The ionization constant of propanoic acid is $1.32 \times 10^{-5}$. Calculate the degree of ionization of the acid in its $0.05 \mathrm{M}$ solution and also its pH. What will be its degree of ionization if the solution is $0.01 \mathrm{M}$ in $\mathrm{HCl}$ also?
Let the degree of ionization of propanoic acid be α.
Then, representing propionic acid as HA, we have:
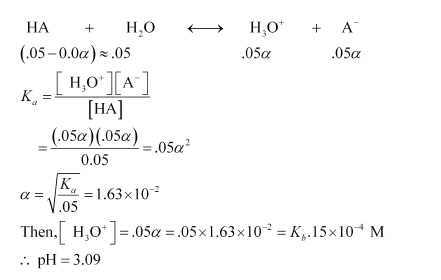
In the presence of 0.1M of HCl, let α´ be the degree of ionization.
Then, $\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=0.01$
$\left[\mathrm{A}^{-}\right]=005 \alpha^{\prime}$
$[\mathrm{HA}]=.05$
$K_{a}=\frac{0.01 \times .05 \alpha^{\prime}}{.05}$
$1.32 \times 10^{-5}=.01 \times \alpha^{\prime}$
$\alpha^{\prime}=1.32 \times 10^{-3}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
