Question:
The process that is NOT endothermic in nature is :
Correct Option: , 2
Solution:
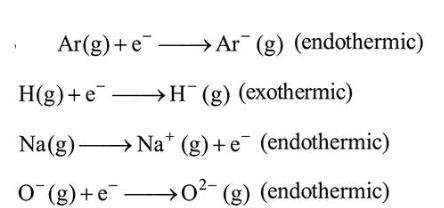
- Electron gaining enthalpy (EGE) of $\mathrm{H}(\mathrm{g})$ is negative while that of $\operatorname{Ar}(\mathrm{g})$ is positive due to $n s^{2} n p^{6}$ configuration.
- Second EGE is always positive for an atom.
- Ionization potential of an atom is positive.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
