The reaction of white phosphorus on boiling with alkali in inert atmosphere resulted in the formation of product 'A'.
Question:
The reaction of white phosphorus on boiling with alkali in inert atmosphere resulted in the formation of product 'A'. The reaction $1 \mathrm{~mol}$ of 'A' with excess of $\mathrm{AgNO}_{3}$ in aqueous medium gives_______________ $\operatorname{mol}(\mathrm{s})$ of $\mathrm{Ag}$. (Round off to the Nearest Integer).
Solution:
(4)
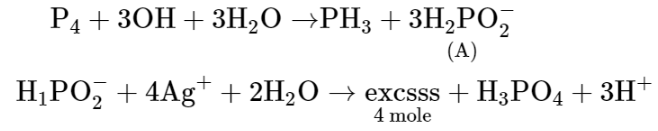
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
