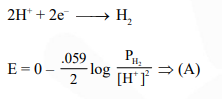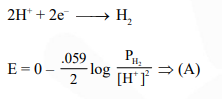Question: The reduction potential of hydrogen half-cell will be negative if :-
$\mathrm{p}\left(\mathrm{H}_{2}\right)=2 \operatorname{atm}\left[\mathrm{H}^{+}\right]=1.0 \mathrm{M}$
$\mathrm{p}\left(\mathrm{H}_{2}\right)=2 \mathrm{~atm}$ and $\left[\mathrm{H}^{+}\right]=2.0 \mathrm{M}$
$\mathrm{p}\left(\mathrm{H}_{2}\right)=1 \mathrm{~atm}$ and $\left[\mathrm{H}^{+}\right]=2.0 \mathrm{M}$
$\mathrm{p}\left(\mathrm{H}_{2}\right)=1 \mathrm{~atm}$ and $\left[\mathrm{H}^{+}\right]=1.0 \mathrm{M}$
Correct Option:
Solution: