Question:
The type of hybridisation and magnetic property of the complex $\left[\mathrm{MnCl}_{6}\right]^{3-}$, respectively, are :
Correct Option: , 4
Solution:
$\left[\mathrm{MnCl}_{6}\right]^{3-}$
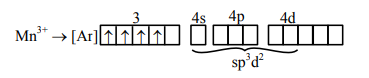
Paramagnetic and having 4 unpaired electrons.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
