The values of $\mathrm{K}_{\mathrm{p}} / \mathrm{K}_{\mathrm{c}}$ for the following reactions at 300 $\mathrm{K}$ are, respectively: (At $300 \mathrm{~K}, \mathrm{RT}=24.62 \mathrm{dm}^{3} \mathrm{~atm}$ $\mathrm{mol}^{-1}$ )
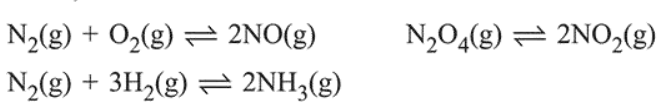
Correct Option: , 2
$\mathrm{K}_{\mathrm{p}}=K_{c}(\mathrm{RT})^{\Delta n_{g}}$
$\Delta \mathrm{n}_{\mathrm{g}}=$ No. of gaseous moles of products
- No. of gaseous moles of reactants
$\frac{K_{p}}{K_{c}}=(\mathrm{RT})^{\Delta^{n} g}$
$\mathrm{N}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NO}(\mathrm{g}), \Delta n_{g}=0$
$=\left(24.62 \mathrm{dm}^{3} \mathrm{~atm} \mathrm{~mol}^{-1}\right)^{0}=1$
$\mathrm{N}_{2} \mathrm{O}_{4}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NO}_{2}(\mathrm{~g}), \Delta n_{g}=1$
$\frac{K_{p}}{K_{c}}=24.62 \mathrm{dm}^{3} \mathrm{~atm} \mathrm{~mol}^{-1}$
$\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g}), \Delta n_{g}=-2$
$\frac{K_{p}}{K_{c}}=\left(24.62 \mathrm{dm}^{-6} \mathrm{~atm}^{-2} \mathrm{~mol}^{-2}\right)^{-2}$
$=\frac{1}{\left(24.62 \mathrm{dm}^{2} \mathrm{~atm} \mathrm{~mol}^{-1}\right)^{2}}$
$=1.65 \times 10^{-3} \mathrm{dm}^{-6} \mathrm{~atm}^{-2} \mathrm{~mol}^{-2}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
