Question:
The variation of equilibrium constant with temperature is given below :
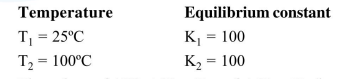
The values of $\Delta \mathrm{H}^{\circ}, \Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{1}$ and $\Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{2}$ (in $\mathrm{kJ} \mathrm{mol}^{-1}$ ) respectively, are close to
$\left[\right.$ Use $\left.\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\right]$
Correct Option: , 3
Solution:
(3) $28.4,-5.71$ and $-14.29$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
