Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
A bucket full of water is placed in a room at $15^{\circ} \mathrm{C}$ with initial relative humidity $40 \%$. The volume of the room is $50 \mathrm{~m}^{3}$. (a) How much water will evaporate? (b) If the room temperature is increased by $5^{\circ} \mathrm{C}$ how much more water will evaporate? The saturation vapour pressure of water at $15^{\circ} \mathrm{C}$ and $20^{\circ} \mathrm{C}$ are $1.6 \mathrm{kPa}$ and $2.4 \mathrm{kPa}$ respectively.
Solution:
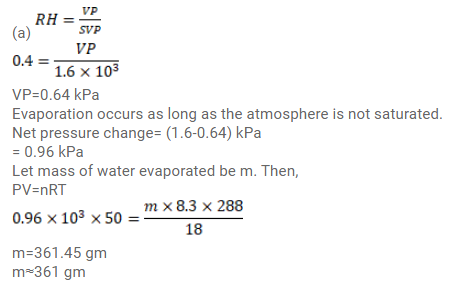
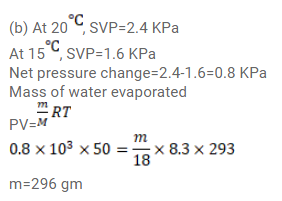
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
