Question.
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from an energy level with n = 4 to an energy level with n = 2?
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from an energy level with n = 4 to an energy level with n = 2?
Solution:
The ni = 4 to nf = 2 transition will give rise to a spectral line of the Balmer series. The energy involved in the transition is given by the relation,
$E=2.18 \times 10^{-18}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{j}^{2}}\right]$
Substituting the values in the given expression of E
$E=2.18 \times 10^{-18}\left[\frac{1}{4^{2}}-\frac{1}{2^{2}}\right]$
$=2.18 \times 10^{-18}\left[\frac{1-4}{16}\right]$
$=2.18 \times 10^{-18} \times\left(-\frac{3}{16}\right)$
$\left.E=-\left(4.0875 \times 10^{-19}\right]\right)$
$\left.E=-\left(4.0875 \times 10^{-19}\right]\right)$
Wavelength of light emitted $\quad(\lambda)=\frac{\text { hc }}{E}$
$\left(\right.$ since $\left.E=\frac{h c}{\lambda}\right)$
Substituting the values in the given expression of $\lambda:$
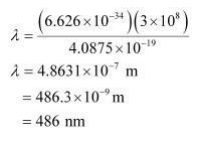
The ni = 4 to nf = 2 transition will give rise to a spectral line of the Balmer series. The energy involved in the transition is given by the relation,
$E=2.18 \times 10^{-18}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{j}^{2}}\right]$
Substituting the values in the given expression of E
$E=2.18 \times 10^{-18}\left[\frac{1}{4^{2}}-\frac{1}{2^{2}}\right]$
$=2.18 \times 10^{-18}\left[\frac{1-4}{16}\right]$
$=2.18 \times 10^{-18} \times\left(-\frac{3}{16}\right)$
$\left.E=-\left(4.0875 \times 10^{-19}\right]\right)$
$\left.E=-\left(4.0875 \times 10^{-19}\right]\right)$
Wavelength of light emitted $\quad(\lambda)=\frac{\text { hc }}{E}$
$\left(\right.$ since $\left.E=\frac{h c}{\lambda}\right)$
Substituting the values in the given expression of $\lambda:$
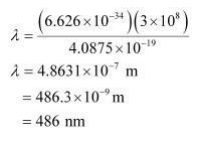
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
