Question:
Which amongst the following is the strongest acid?
Correct Option: , 3
Solution:
Due to the resonance stabilisation of the conjugate base, $\mathrm{CH}(\mathrm{CN})_{3}$ is the strongest acid amongst the given compounds.
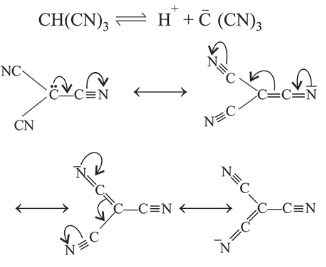
The conjugate bases of $\mathrm{CHBr}_{3}$ and $\mathrm{CHl}_{3}$ are stabilised by inductive effect of halogens. This is why, they are less stable. Also, the conjugate base of $\mathrm{CHCl}_{3}$ involves back-bonding between $2 p$ and $3 p$ orbitals
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
