Which one of the following species responds to an external magnetic field?
Correct Option: 1,
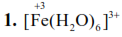
$\mathrm{Fe}^{3+}:[\mathrm{Ar}] 3 \mathrm{~d}^{5}$
Hybridisation : $\mathrm{sp}^{3} \mathrm{~d}^{2}$
Magnetic nature : Paramagnetic (so this complex response to external magnetic field)
2. $\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}$
$\mathrm{Ni}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^{8}$
Hybridisation : dsp $^{2}$
Magnetic nature : diamagnetic
3. $\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}$
$\mathrm{Co}^{3+}:[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
Hybridisation : $d^{2} s p^{3}$
Magnetic nature : diamagnetic
$\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]$
$\mathrm{Ni}:[\mathrm{Ar}] 3 \mathrm{~d}^{8} 4 \mathrm{~s}^{2}$
Hybridisation : $\mathrm{sp}^{3}$
Magnetic nature : diamagnetic
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
