Question.
Why are decomposition reactions called the opposite of combination reactions ? Write equations for these reactions.
Why are decomposition reactions called the opposite of combination reactions ? Write equations for these reactions.
solution:
Decomposition reactions are those in which a compound breaks down to form two or more substances. These reactions require a source of energy to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
$\mathrm{AB}+$ Energy $\longrightarrow \mathrm{A}+\mathrm{B}$
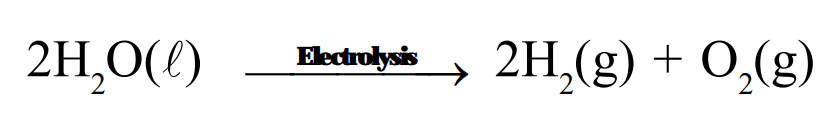
Combination reaction
$\mathrm{A}+\mathrm{B} \longrightarrow \mathrm{AB}+$ Energy
$2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}(\ell)+$ Energy
Decomposition reactions are those in which a compound breaks down to form two or more substances. These reactions require a source of energy to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
$\mathrm{AB}+$ Energy $\longrightarrow \mathrm{A}+\mathrm{B}$
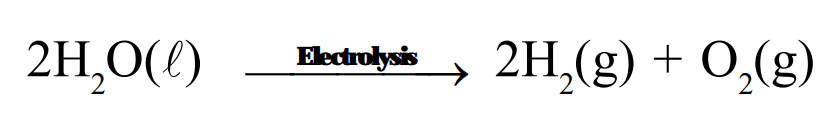
Combination reaction
$\mathrm{A}+\mathrm{B} \longrightarrow \mathrm{AB}+$ Energy
$2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}(\ell)+$ Energy
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
