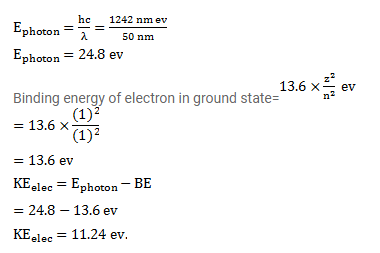
Question:
A hydrogen atom in ground state absorbs a photon of ultraviolet radiation of wavelength $50 \mathrm{~nm}$. Assuming that the entire photon energy is taken up by the electron, with what kinetic energy will the electron be ejected?
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
