Get Co-ordination Compounds important questions for Boards exams. Download or View the Important Question bank for Class 11 Chemistry. These important questions will play significant role in clearing concepts of Chemistry. This question bank is designed by NCERT keeping in mind and the questions are updated with respect to upcoming Board exams. You will get here all the important questions for class 12 chemistry chapter wise CBSE.
Click Here for Detailed Chapter-wise Notes of Chemistry for Class 12th, JEE & NEET.
You can access free study material for all three subject’s Physics, Chemistry and Mathematics.
Click Here for Detailed Notes of any chapter.
eSaral provides you complete edge to prepare for Board and Competitive Exams like JEE, NEET, BITSAT, etc.
We have transformed classroom in such a way that a student can study anytime anywhere. With the help of AI we have made the learning Personalized, adaptive and accessible for each and every one.
Visit eSaral Website to download or view free study material for JEE & NEET.
Also get to know about the strategies to Crack Exam in limited time period.
Q. What is the coordination number of central metal ion in $\left[\mathrm{Fe}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-2}$
Ans. The coordination number of central metal ion is 6.
Q. Define a ‘ligand’. Give an example also.
Ans. Ligand is an atom or group of atoms which is either negatively charged or have lone pair of electrons to form co-ordinate bond with central metal ion.
Q. What is oxidation state of $\mathrm{Co}$ in complex $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{NO}_{2}\right) \mathrm{Cl}\right]$ $\left[A u(C N)_{3}\right] ?$
[AI 1997C]
Ans. Cobalt has $+3$ oxidation state. $[x+0-1-1+1-2=0] \Rightarrow x=3$
Q. Write the name of bidentate ligand.
Ans. Ethylene diamine, oxalate ion.
Q. What is the charge on the complex of $P t^{2+}$ in which the ligands are-one water molecule, one pyridine molecule and one ethylenediamine molecule?
Ans. $\left[P t\left(H_{2} O\right) p y(e n)\right]^{2+} .$ The charge on complex ion is $+2$ because ligands are neutral molecules.
Q. Write IUPAC name of the complex $N a_{3}\left[C r(O H)_{z} F_{4}\right] .$
[Delhi 2003]
Ans. Sodium tetrafluorodihydroxochromate (III)
Q. Fill in the blanks :
The complexes with coordination number 6 having three ......................... ligands are optically active.
[CBSE Sample Paper 1997]
Ans. Different.
Q. Give the chemical formula of potassium hexacyano ferrate $(\mathbb{I})$
Ans. $K_{4}\left[F e(C N)_{6}\right]$
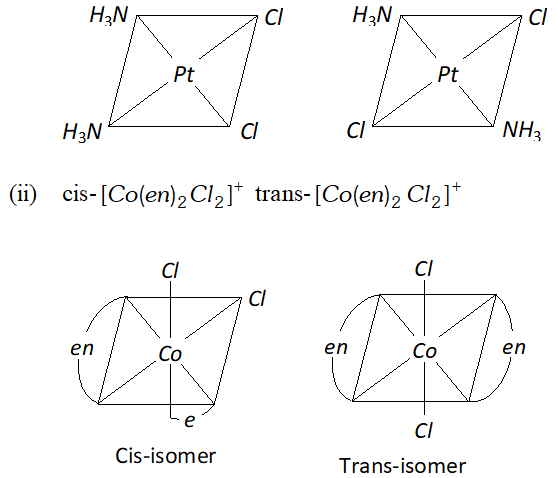
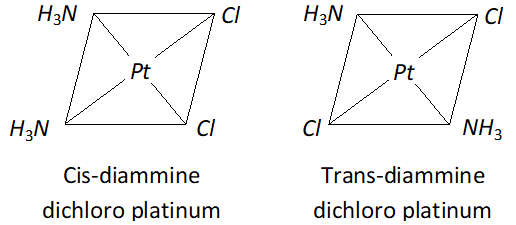
Q. Write the structures of a pair of complexes showing geometrical isomerism.
Ans. (i) $\operatorname{cis}-\left[P t\left(N H_{3}\right)_{2} C l_{2}\right],$ trans-

Q. Write ionisation isomer of $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right]^{2+} \mathrm{SO}_{4}^{2-}$
Ans. $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{SO}_{4}\right]^{+} \mathrm{Br}^{-} \quad$ is ionisation isomer of
$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right]^{2+} \mathrm{SO}_{4}^{2-}$
Q. Name the type of isomerism that occurs in complexes in which both cation and anion are complex ions.
Ans. Co-ordination isomerism.
Q. What scheme of hybridisation is proposed for $C o$ in $\left[C O\left(N H_{3}\right)_{6}\right]^{3+9} ?$
[AI 1999C]
Ans. $d^{2} s p^{3}$
Q. What is the shape of $\left[N i(C N)_{4}\right]^{2-} ?$
[CBSE Sample Paper 1997]
Ans. It is square planar.
Q. Name the hybridisation and the orbitals involved in the shape of $\left[N i(C N)_{4}\right]^{2-}$
Ans. - The hybridization is $d s p^{2}$ and shape is square planar.
Q. Which of the two is more stable $K_{4}\left[F e(C N)_{6}\right]$ or $K_{3}\left[\text { Fe }(C N)_{6}\right] ?$
Ans. $K_{3}\left[F e(C N)_{6}\right]$ is more stable than $K_{4}\left[F e(C N)_{6}\right]$
Q. Give one use of Ziegler Natta catalyst.
Ans. It is used as catalyst in polymerisation of alkanes as heterogeneous catalyst.
Q. Name the metal present in (i) Chlorophyll (ii) Haemoglobin
(iii) Vitamin $\mathrm{B}_{12}$ (iv) Cis-platin.
Ans. (i) $\mathrm{Mg}$ (ii) Fe (iii) Co (iv) Pt.
Q. What is meant by hexadentate ligand? Give one example. How is such a ligand useful for measuring hardness of water?
Ans. Hexadentate ligand is a ligand which has 6 donor atoms, e.g. EDTA
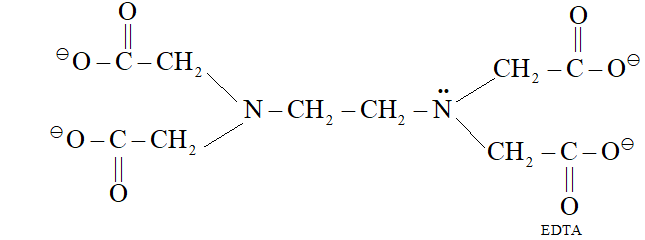 EDTA forms complex with $C a^{2+}$ and $M g^{2+},$ therefore, it is used for measuring hardness of water.
EDTA forms complex with $C a^{2+}$ and $M g^{2+},$ therefore, it is used for measuring hardness of water.
 EDTA forms complex with $C a^{2+}$ and $M g^{2+},$ therefore, it is used for measuring hardness of water.
EDTA forms complex with $C a^{2+}$ and $M g^{2+},$ therefore, it is used for measuring hardness of water.
Q. Define coordination number. What is the coordination number of central metal atom in
(i) $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2}$
(ii) $K_{2}\left[F e C l_{4}\right]$
Ans. Coordination number is defined as number of monodentate ligands surrounding a central metal atom or ion. The coordination number of $\mathrm{Co}$ in ( i ) is 6 and $\mathrm{Fe}$ in ( i ) is 4.
Q. Explain geometrical isomerism with reference to square planar complexes giving one example. How is tetrahedral complexes with simple ligands do not exhibit geometrical isomerism?
Ans. Explain geometrical isomerism with reference to square planar complexes giving one example. How is tetrahedral complexes with simple ligands do not exhibit geometrical isomerism?
 Tetrahedral complexes do not show geometrical isomerism because relative position of ligands are the same.
Tetrahedral complexes do not show geometrical isomerism because relative position of ligands are the same.
 Tetrahedral complexes do not show geometrical isomerism because relative position of ligands are the same.
Tetrahedral complexes do not show geometrical isomerism because relative position of ligands are the same.
Q. In a complex ion : [Co(NH3)3(H2O)2Cl]+
(a) Identify the ligand’s formula and the charge on each one of them and
(b) Write the geometry of complex ion.
Ans. (a) Ligands are $N H_{3},$ the charge on it is zero. $H_{2} O$ has zero charge. $C l$ has negative charge $(-1)$
(b) It has octahedral shape.
Q. Square planar complexes with a coordination number 4 exhibit geometrical isomerism whereas tetrahedral complexes do not, why ?
Ans. The tetrahedral complexes do not show geometrical isomerism because the relative positions of the atoms with respect to each other will be same. The square planar complexes on the other hand show this kind of isomerism because if same kind of ligands occupy position adjacent to each other, it is called cis-form and if these are opposite to each other, it is called trans-form.
Q. How is the magnitude of $\Delta_{0}$ affected by (i) nature of ligand and (ii) oxidation state of metal ion?
[Delhi 2004]
Ans. (i) $\quad \Delta_{0}$ is affected by nature of ligand. Greater the strength of ligand, greater the value of
(ii) is also affected by oxidation state of central metal ion. Generally, the higher the ionic charge on the central metal
ion, the greater will be the value of $\Delta_{0}$.
Q. Using the valence bond approach, deduce the shape and magnetic character of $\left[\mathrm{Cr}(\mathrm{CO})_{6}\right]$
$[\text { At. no. of } C r=24]$
[Delhi 2003]
Ans. Cr has electronic configuration
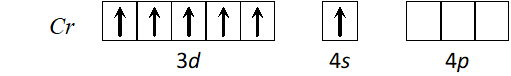 $C r(0)$ has electronic configuration $[A r] 4 s^{0} 3 d^{6} \because C O$ will cause pairing of electrons.
$C r(0)$ has electronic configuration $[A r] 4 s^{0} 3 d^{6} \because C O$ will cause pairing of electrons.
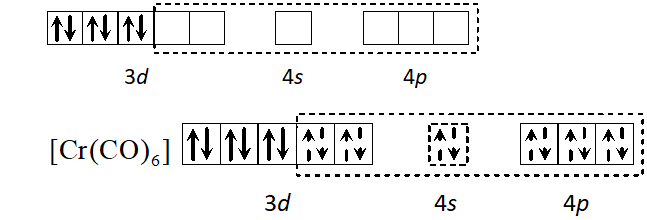 $d^{2} s p^{3}$ hybridisation octahedral shape, diamagnetic.
$d^{2} s p^{3}$ hybridisation octahedral shape, diamagnetic.
 $d^{2} s p^{3}$ hybridisation octahedral shape, diamagnetic.
$d^{2} s p^{3}$ hybridisation octahedral shape, diamagnetic.
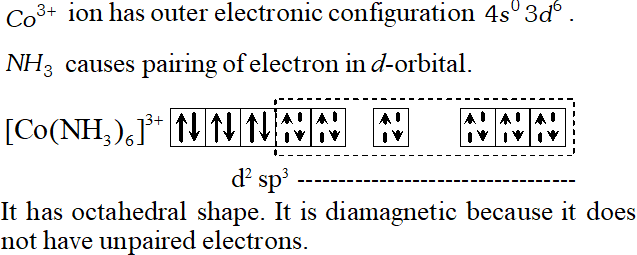
Q. Using valence bond theory of complexes, explain the geometry and diamagnetic nature of the ion $\left[C o\left(N H_{3}\right)_{6}\right]^{3+}$ [At. no. of $C o=27]$
[Delhi 2001]
Ans. $\mathrm{Co}(27) 1 \mathrm{s}^{2} 2 \mathrm{s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{s}^{2} 3 \mathrm{p}^{6} 4 \mathrm{s}^{2} 3 \mathrm{d}^{7}$

Q. Among $\left[A g\left(N H_{3}\right)_{2}\right] C l,\left[N i(C N)_{4}\right]^{2-}$ and $\left[C u C l_{4}\right]^{2-}$ which
(i) Has square planar geometry?
(ii) Remains colourless in aqueous solutions and why? $[\text { At. no. of } A g=47, N i=28, C u=29]$
Ans. $-\left[N i(C N)_{4}\right]^{2-}$ has square planar geometry.
$\left[A g\left(N H_{3}\right)_{2} C l\right]$ remains colourless in aqueous solution
because $A g^{+}$ has no unpaired electron, therefore it cannot undergo $d-d$ -transition.
Q. Explain the following:
(i) $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$ is diamagnetic, whereas $\left[\mathrm{CoF}_{6}\right]^{3-}$ is paramagnetic.
(ii) $\left[F e\left(H_{2} O\right)_{6}\right]^{3+}$ is more paramagnetic than $\left[F e(C N)_{6}\right]^{3-}$
[AI 2000]
Ans. (i) $\quad\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$ is diamagnetic because $\mathrm{NH}_{3}$ is strong
field ligand, causes pairing of electrons whereas $\left[\mathrm{CoF}_{6}\right]^{3-}$ is
paramagnetic because $F^{-}$ is weak field ligand, does not cause pairing of electrons and there are unpaired electrons.
(ii) $\left[F e\left(H_{2} O\right)_{6}\right]^{3+}$ has more unpaired electron than
$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-},$ therefore, more paramagnetic.
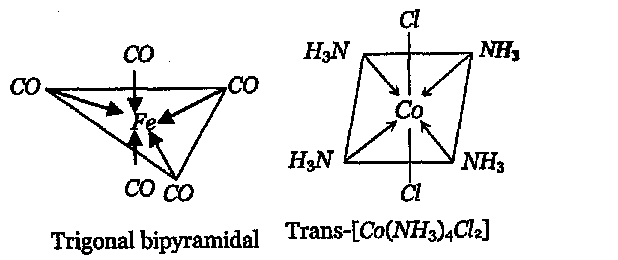
Q. Draw the structures and write the hybridised state of the central atom of each of the following species:
(i) $\mathrm{Fe}(\mathrm{CO})_{5}$ (ii) trans- $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]^{+}$
$[\text { At. no. of } F e=26, C o=27]$
[AI 1999]
Ans.

Q. Describe the shape of tetra cyano nicklate (II) ion and account for its magnetic property.
[AI 1996; CBSE Sample Paper 1997]
Ans. $\left[N i C N_{4}\right]^{2-}, C N^{-}$ cause pairing of electrons in $N i^{2+}$ ion.
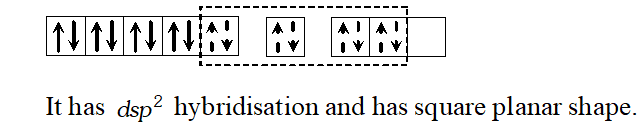

Q. Explain how $\left[P t\left(N H_{3}\right) C l_{2}\right]$ and $\left[P t\left(N H_{3}\right)\right] C l_{2}$ will differ in their electrolytic conductances. Give the hybridisation state of $P t$ in these compounds. [At. no. of Pt is $78]$
Ans. Ionization isomerism and Geometrical isomerism is exhibited
by $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Br}$
The ionisation isomers are $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Br}$ and
$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl} \mathrm{Br}\right] \mathrm{Cl}$ The geometrical isomers are
 The central atom has $d^{2} s p^{3}$ hybridization.
The central atom has $d^{2} s p^{3}$ hybridization.
 The central atom has $d^{2} s p^{3}$ hybridization.
The central atom has $d^{2} s p^{3}$ hybridization.
Q. How is stability of coordination compounds determined in aqueous solution?
Ans. Stability of co-ordination compound in aqueous solution is determined with the help of stability constant. Higher the value of stability constant, greater will be stability. Smaller the cation, higher the charge on the cation, more stable will be the complex.
Q. Explain why a chelating complex is more stable than unchelated complex.
Ans. Chelating complex is more stable than unchelated complex because there is strong force of attraction between cation and polydentate ligand as compared to monodentate ligand.
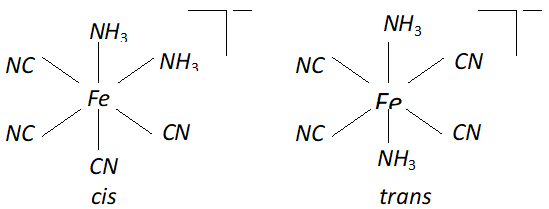
Q. Draw structures of geometrical isomers of $\left[F e\left(N H_{3}\right)_{2}(C N)_{4}\right]^{-}$
Ans.

Q. (i) Co-ordination compound has the formula $\mathrm{CoCl}_{3}, 4 \mathrm{NH}_{3}$ It does not liberate ammonia but forms a precipitate with $A g N O_{3} .$ Write the structure and IUPAC name of the complex compound.
(ii) Name a ligand which is bidentate and give an example of the complex formed by this ligand.
[AI 1998]
Ans. $\begin{array}{llll}{-(1)} & {\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Cl}} & {\text { Its }} & {\text { IUPAC }} & {\text { name }} & {\text { is }}\end{array}$
tetraamminedichlorocobalt (III) chloride.
(ii) Ethylene diamine (en) is bidentate ligand. $\left[\mathrm{Co}(e n)_{3}\right]^{3+} .$ Its IUPAC name is tris (ethylene diamine) cobalt (MI) ion.
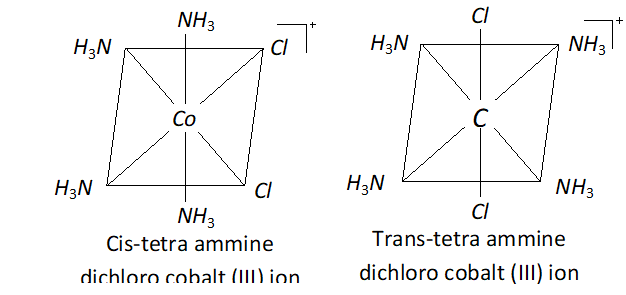
Q. Write the IUPAC name of $\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right] \mathrm{Cl}$ and draw the structures of all the isomers with this formula of complex.
[Delhi 2004]
Ans. The IUPAC name is Dichlorobis (ethylene diamine) cobalt (III) chloride.
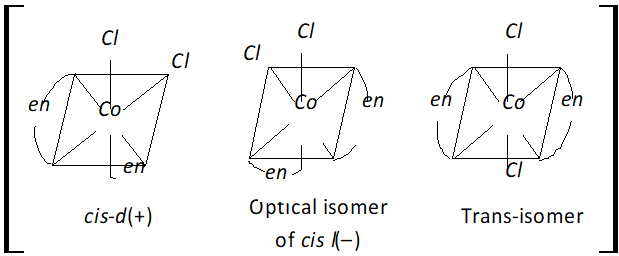

Q. Answer the following:
(i) Differentiate between a bidentate ligand and a monodentate ligand.
(ii) Write the IUPAC name of $\left[P t\left(N H_{3}\right)_{2} C l_{2}\right] C l_{2}$
(iii) Draw the structures of geometrical isomers of
$$ \left[\mathrm{Co}\left(\mathrm{NH}_{4}\right)_{4} \mathrm{Cl}_{2}\right]^{+} $$
[AI 2003C]
Ans. (i) Bidentate ligand has two donor atoms which can form two sigma bonds whereas monodentate ligand can form only one bond
(ii) Diammine dichloro platinum (IV) chloride.
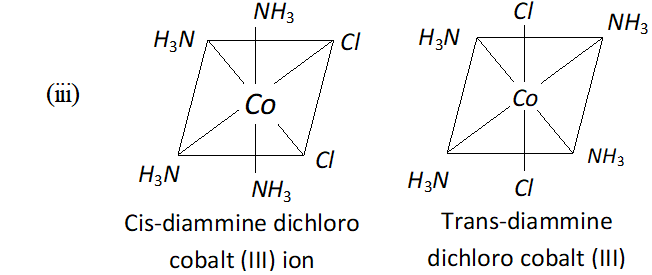

Q. Explain each of the following observations:
(i) Tetrahedral $N i$ (II) complexes are paramagnetic but square planar $N i$ (II) complexes are diamagnetic.
(ii) Only transition metals are known to form $\pi$ -complexes.
[Delhi 2002]
Ans. $-(1) \quad$ Tetrahedral complexes of $N i$ (II) are paramagnetic due to presence of unpaired electrons whereas square planar complexes of $N i^{2+}$ are diamagnetic due to absence of unpaired electrons.
(ii) Iransition metals have vacant $d$ -orbitals as well as pair of electrons in $d$ -orbitals, therefore, they can form $\pi$ bonded complexes.
Q. Mention the geometrical shapes attained by the following type of hybrid orbitals:
(i) $S p^{3}$
(ii) $d s p^{2}$
(iii) $d^{2} s p^{3}$
Give an example of each of the above.
Ans. (i) $s p^{3}$ has tetrahedral shape, e.g., $\left[Z n\left(N H_{3}\right)_{4}\right]^{2+}$ is hybridised.
(ii) has square planar, e.g., is hybridised.
(iii) has octahedral shape, e.g., is hybridised.
Q. (i) Name two main factors that favour a metal ion's forming complex.
(ii) Give an example of industrial application of formation of coordination complex.
(iii) Write the IUPAC name of $\left[\mathrm{Co}(e n)_{2} \mathrm{Cl}(\mathrm{ONO})\right]^{+}$
[AI 2001C]
Ans. (i) $\quad$ (a) small size of cation and higher charge.
(b) presence of vacant $d$ -orbitals.
(ii) $\quad K\left[A g(C N)_{2}\right]$ is used for silver plating and $K\left[A u(C N)_{2}\right]$ is used for gold plating.
(iii) Chlorobis (ethylene diamine) nitrito cobalt (III) ion.
Q. Formation of complex is exothermic or endothermic process. Explain why. What is the effect of temperature on stability of complex compounds ?
Ans. Formation of complex is an exothermic process because there is force of attraction between central metal ion and ligands.
Stability of complex decreases with the increases in temperature because formation of complex is exothermic process. On heating, coordination bond between central metal ion and ligand will break.
Q. Example of synergic bonding interactions in a carbonyl complex.
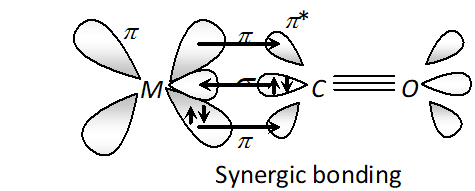
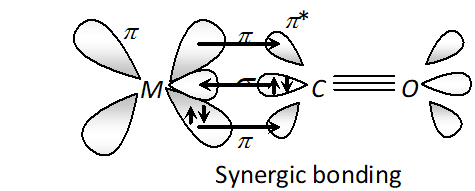
Ans. The metal-carbon bond in metal carbonyls possess both $s$ and $p$ character. The $M-C \sigma$ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. The $M-C \pi$ -bond is formed by the donation of a pair of electrons from a filled $d$ -orbital of metal into the vacant antibonding $\pi^{*}$ orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between $C O$ and the metal.
Q. The hexaquomanganese (II) ion contains five unpaired electrons while the hexacyano ion contains only one unpaired electron. Explain using Crystal Field Theory.
Ans. $-M n$ in $+2$ state has the configuration $3 d^{5} .$ In presence of $H_{2} O$ as ligand, the distribution of these five electrons is $t_{2 g}^{3} e_{g}^{2},$ i.e., all the electrons remain unpaired. In presence of $\mathrm{CN}^{-}$ as ligand, the distribution is $t_{2 g}^{5} e_{g}^{0},$ i.e. , two $t_{2 g}$ orbitals contain paired electrons while the third orbital contains one unpaired electron.
Q. Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans. The main postulates of Werner's theory of coordination compounds are as follows:
(i) Metal possess two types of valences called
(a) primary valence which are ionisable.
(b) secondary valence which are non-ionisable.
(ii) Primary valency is satisfied by the negative ions and it is that which a metal exhibits in the formation of its simple salts. For example, in the coordination compound, $\mathrm{CoCl}_{3} \cdot 6 \mathrm{NH}_{3}$ valency between $\mathrm{Co}$ and $\mathrm{Cl}$ is primary valency and valency between $\mathrm{Co}$ and $\mathrm{NH}_{3}$ is secondary. In terms of modern theories based on electronic configuration, the primary valency is now referred to as "oxidation state' of metal.
(iii) Secondary valencies are satisfied by neutral ligand or negative ligand and are those which metal exercises in the formation of its complex ions. Every cation has a fixed number of secondary valencies and are directed in space about central metal ion in certain fixed directions. The six valencies are regarded as directed to corners of a regular octahedron whereas four may be arranged either in square planar manner or tetrahedral manner. This explains the shape of coordination compound (or complex ion or compound).
For example, in coordination compound $\left[\mathrm{CoCl}_{3} \cdot 6 \mathrm{NH}_{3}\right],$ six ammonia molecules linked to $\mathrm{Co}$ by secondary valencies are directed to six corners of a regular octahedron and thus account for structure of as follows:
Q. Using IUPAC norms write the formulas for the following
(i) Tetrahydroxozincate (II)
(ii) Hexaamminecobalt (III) sulphate
(iii) Potassium tetrachloridopalladate (II)
(iv) Potassium tri (oxalato) chromate (III)
(v) Diaminedichloridoplatinum(II)
(vi) Hexaammineplatinum(IV)
Ans.
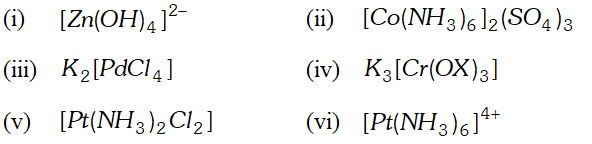
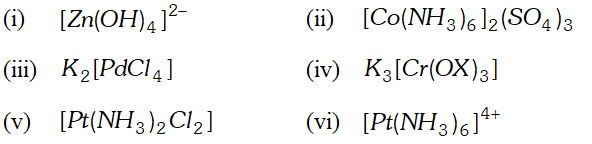
Q. Using IUPAC norms write the systematic names of the following:
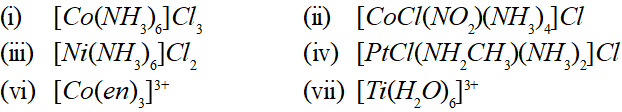
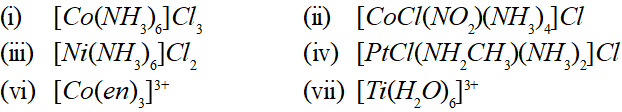
Ans. (i) Hexammine cobalt (III) chloride
(ii) Tetrammine chloronitro cobalt (III) chloride
(iii) Hexammine nickel (II) chloride
(iv) Diamminechloro (methylamine) platinum (II) chloride
(vi) Tris $(1,2$ -ethane diamine) cobalt (III) ion
(vii) Hexaqua titanium (III) ion
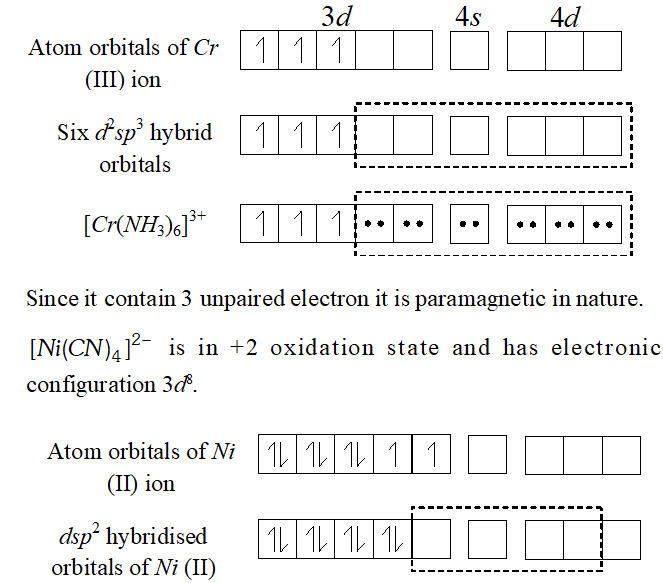
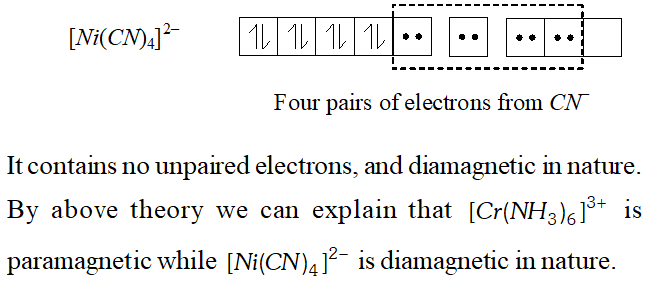
Q. $\left[C r\left(N H_{3}\right)_{6}\right]^{3+}$ is paramagnetic while $\left[N i(C N)_{4}\right]^{2-}$ is diamagnetic. Explain why?
Ans. In $\left[C r\left(N H_{3}\right)_{6}\right]^{3+},$ the chromium ion is in $+3$ oxidation state and has electronic configuration of $3 d^{\beta},$ as shown below :


Q. List various types of isomerism possible for coordination compounds, given an example of each.
Ans. Two principal types of isomerism are known among coordination compounds:
(i) Stereo isomerism:
(a) Geometrical isomerism, (b) Optical isomerism
(ii) Structural isomerism:
(a) Linkage isomerism, (b) Coordination isomerism, (c) Ionisation isomerism, (d) Solvate isomerism
(i) Stereo isomerism:
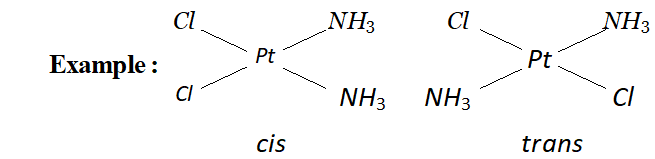
(a) Geometrical isomerism: This type of isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands.
For example, in a square planar complex of formula $\left[M X_{2} L_{2}\right]$ the two ligands $X$ may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer.
 (b) Optical isomerism: Optical isomers are mirror images that cannot be super imposed on one another. There are called as enantiomers. The two forms are called dextro (d) and laevo $(l)$ depending upon direction they rotate the plane of polarised light in polarimeter.
(b) Optical isomerism: Optical isomers are mirror images that cannot be super imposed on one another. There are called as enantiomers. The two forms are called dextro (d) and laevo $(l)$ depending upon direction they rotate the plane of polarised light in polarimeter.
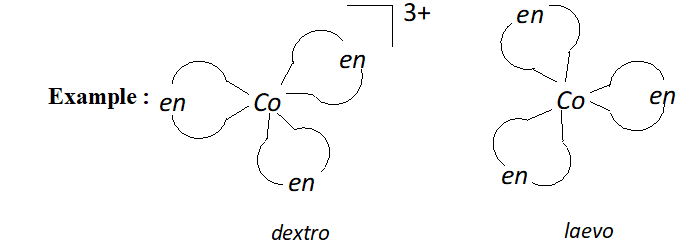 Optical isomers $(d \text { and } l)$ of $\left[C o(e n)_{3}\right]^{3+}$
(ii) Structural isomerism:
(a) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocynate ligand, $N C S^{-}$ which may find through the nitrogen to give $M \leftarrow N C S$ or through sulphur to give $M \leftarrow S C N$
(b) Coordination isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]$ and $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Co}(\mathrm{CN})_{6}\right]$
(c) Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become counter ion.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{SO}_{4}\right] \mathrm{Br}$ and $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right] \mathrm{SO}_{4}$
(d) Solvate isomerism: This form of isomerism is known as "hydrate isomerism'. Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
Example: $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3} \&\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$
Optical isomers $(d \text { and } l)$ of $\left[C o(e n)_{3}\right]^{3+}$
(ii) Structural isomerism:
(a) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocynate ligand, $N C S^{-}$ which may find through the nitrogen to give $M \leftarrow N C S$ or through sulphur to give $M \leftarrow S C N$
(b) Coordination isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]$ and $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Co}(\mathrm{CN})_{6}\right]$
(c) Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become counter ion.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{SO}_{4}\right] \mathrm{Br}$ and $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right] \mathrm{SO}_{4}$
(d) Solvate isomerism: This form of isomerism is known as "hydrate isomerism'. Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
Example: $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3} \&\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$
 (b) Optical isomerism: Optical isomers are mirror images that cannot be super imposed on one another. There are called as enantiomers. The two forms are called dextro (d) and laevo $(l)$ depending upon direction they rotate the plane of polarised light in polarimeter.
(b) Optical isomerism: Optical isomers are mirror images that cannot be super imposed on one another. There are called as enantiomers. The two forms are called dextro (d) and laevo $(l)$ depending upon direction they rotate the plane of polarised light in polarimeter.
 Optical isomers $(d \text { and } l)$ of $\left[C o(e n)_{3}\right]^{3+}$
(ii) Structural isomerism:
(a) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocynate ligand, $N C S^{-}$ which may find through the nitrogen to give $M \leftarrow N C S$ or through sulphur to give $M \leftarrow S C N$
(b) Coordination isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]$ and $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Co}(\mathrm{CN})_{6}\right]$
(c) Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become counter ion.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{SO}_{4}\right] \mathrm{Br}$ and $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right] \mathrm{SO}_{4}$
(d) Solvate isomerism: This form of isomerism is known as "hydrate isomerism'. Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
Example: $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3} \&\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$
Optical isomers $(d \text { and } l)$ of $\left[C o(e n)_{3}\right]^{3+}$
(ii) Structural isomerism:
(a) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocynate ligand, $N C S^{-}$ which may find through the nitrogen to give $M \leftarrow N C S$ or through sulphur to give $M \leftarrow S C N$
(b) Coordination isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]$ and $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Co}(\mathrm{CN})_{6}\right]$
(c) Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become counter ion.
Example : $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{SO}_{4}\right] \mathrm{Br}$ and $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5} \mathrm{Br}\right] \mathrm{SO}_{4}$
(d) Solvate isomerism: This form of isomerism is known as "hydrate isomerism'. Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
Example: $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3} \&\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$
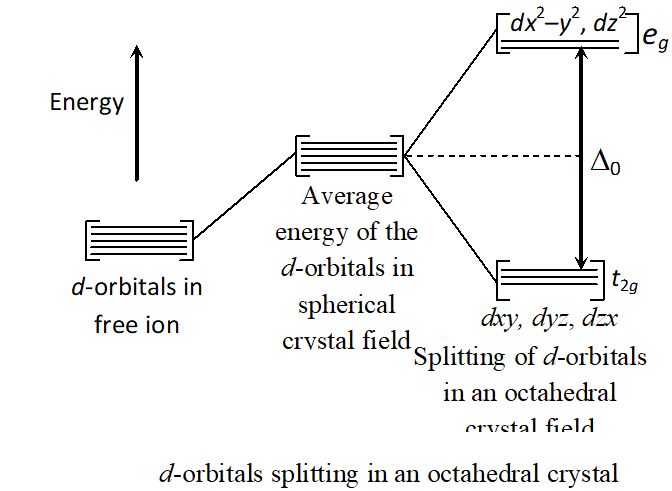
Q. Draw a figure to show splitting of degenerate $d$ -orbital in an octahedral crystal field. How does the magnitude of D_{o} decide the actual configuration of $d$ -orbitals in a complex entity?
[Delhi 2004]
Ans. In $d^{1}$ coordination entity, $d^{1}$ electron occupies $t_{2 g}$ orbitals.
In $d^{2}$ and $d^{3}$ also, electrons occupy orbitals singly in accordance with the Hund's rule. For $d^{4}$ ions two possibility exist. If is less than $P($ energy) required for electron pairing in a single orbital, we have weak field, high spin situation and the fourth electron enters one of the orbitals giving. Fifth electron also enters orbitals, i.e., . When, we have strong field, low spin situation, and pairing will occur in the level with orbitals remain unoccupied in to ions. Greater the value greater the chances of pairing.

Q. What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans. Ligands have different field strengths and as a result the crystal field splitting, $\Delta_{0}$ or $\Delta_{t}$, depends upon the field produced by the ligand and charge on metal ions. Some ligands are able to produce strong fields in which case the splitting will be large whereas others produce weak fields and consequently result in small splitting of $d$ -orbitals. The arrangement of the ligands in order of increasing field strength is known as spectrochemical series.
$I^{-}
$
Q. What is meant by stability of coordination compound in solution? State the factors which govern stability of complexes?
Ans. The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude of the (stability or formation) equilibrium constant for the association, quantitatively expresses the stability. Thus, if we have a reaction of the type:
$M+4 L \rightleftharpoons M L_{4}$
then the larger the stability constant, the higher the proportion of that exists in solution. Free metal ions rarely exist in the solution so that $M$ will usually be surrounded by solvent molecules which will complete with the ligand molecules, $L,$ and be successively replaced by them. For simplicity, we generally ignore these solvent molecules and write four stability constants as follows:
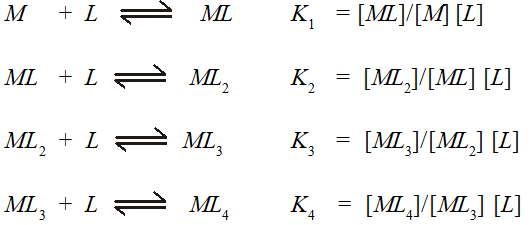 Where $K_{1}, K_{2},$ etc., are referred to as stepwise stability constants. Alternatively, we can write the overall stability constant thus:
$M+4 L \rightleftharpoons M L_{4} \quad \beta_{4}=\left[M L_{4}\right] /[M][L]^{4}$
The stepwise and overall stability constant are therefore related as follows :
$\beta_{4}=K_{1} \times K_{2} \times K_{3} \times K_{4}$ or more generally.
$\beta_{n}=K_{1} \times K_{2} \times K_{3} \times \ldots \ldots \ldots K_{n}$
Factors affecting the stability of complex :
(1) The nature of central ion
(2) The nature of ligand
(1) The nature of central ion : The term nature means the ratio, more is the stability of a complex.
(2) The nature of ligand :
(i) Basic character of ligand : More basic is a ligand, greater is the ease with which it can donate its electrons and therefore more is the stability of complex.
(ii) For charged ligand : The higher the charge and the smaller their size, the more stable are the complexes.
(iii) Chelate effect : Complexes containing chelate ring are usually more stable than similar complexes containing no rings.
Where $K_{1}, K_{2},$ etc., are referred to as stepwise stability constants. Alternatively, we can write the overall stability constant thus:
$M+4 L \rightleftharpoons M L_{4} \quad \beta_{4}=\left[M L_{4}\right] /[M][L]^{4}$
The stepwise and overall stability constant are therefore related as follows :
$\beta_{4}=K_{1} \times K_{2} \times K_{3} \times K_{4}$ or more generally.
$\beta_{n}=K_{1} \times K_{2} \times K_{3} \times \ldots \ldots \ldots K_{n}$
Factors affecting the stability of complex :
(1) The nature of central ion
(2) The nature of ligand
(1) The nature of central ion : The term nature means the ratio, more is the stability of a complex.
(2) The nature of ligand :
(i) Basic character of ligand : More basic is a ligand, greater is the ease with which it can donate its electrons and therefore more is the stability of complex.
(ii) For charged ligand : The higher the charge and the smaller their size, the more stable are the complexes.
(iii) Chelate effect : Complexes containing chelate ring are usually more stable than similar complexes containing no rings.
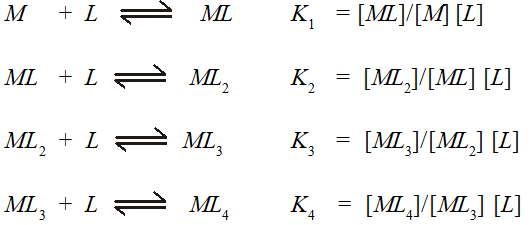 Where $K_{1}, K_{2},$ etc., are referred to as stepwise stability constants. Alternatively, we can write the overall stability constant thus:
$M+4 L \rightleftharpoons M L_{4} \quad \beta_{4}=\left[M L_{4}\right] /[M][L]^{4}$
The stepwise and overall stability constant are therefore related as follows :
$\beta_{4}=K_{1} \times K_{2} \times K_{3} \times K_{4}$ or more generally.
$\beta_{n}=K_{1} \times K_{2} \times K_{3} \times \ldots \ldots \ldots K_{n}$
Factors affecting the stability of complex :
(1) The nature of central ion
(2) The nature of ligand
(1) The nature of central ion : The term nature means the ratio, more is the stability of a complex.
(2) The nature of ligand :
(i) Basic character of ligand : More basic is a ligand, greater is the ease with which it can donate its electrons and therefore more is the stability of complex.
(ii) For charged ligand : The higher the charge and the smaller their size, the more stable are the complexes.
(iii) Chelate effect : Complexes containing chelate ring are usually more stable than similar complexes containing no rings.
Where $K_{1}, K_{2},$ etc., are referred to as stepwise stability constants. Alternatively, we can write the overall stability constant thus:
$M+4 L \rightleftharpoons M L_{4} \quad \beta_{4}=\left[M L_{4}\right] /[M][L]^{4}$
The stepwise and overall stability constant are therefore related as follows :
$\beta_{4}=K_{1} \times K_{2} \times K_{3} \times K_{4}$ or more generally.
$\beta_{n}=K_{1} \times K_{2} \times K_{3} \times \ldots \ldots \ldots K_{n}$
Factors affecting the stability of complex :
(1) The nature of central ion
(2) The nature of ligand
(1) The nature of central ion : The term nature means the ratio, more is the stability of a complex.
(2) The nature of ligand :
(i) Basic character of ligand : More basic is a ligand, greater is the ease with which it can donate its electrons and therefore more is the stability of complex.
(ii) For charged ligand : The higher the charge and the smaller their size, the more stable are the complexes.
(iii) Chelate effect : Complexes containing chelate ring are usually more stable than similar complexes containing no rings.
