JEE Main Previous Year Question of Chemistry with Solutions are available here. Practicing JEE Main Previous Year Papers Questions of Chemistry will help all the JEE aspirants in realizing the question pattern as well as help in analyzing their weak & strong areas.
Get detailed Class 11th &12th Chemistry Notes to prepare for Boards as well as competitive exams like IIT JEE, NEET etc.
eSaral helps the students in clearing and understanding each topic in a better way. eSaral is providing complete chapter-wise notes of Class 11th and 12th both for all subjects.
Besides this, eSaral also offers NCERT Solutions, Previous year questions for JEE Main and Advance, Practice questions, Test Series for JEE Main, JEE Advanced and NEET, Important questions of Physics, Chemistry, Math, and Biology and many more.
Download eSaral app for free study material and video tutorials.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the folloowing statements is correct regarding the behaviour of the solution ?
(1) The solution is non-ideal, showing –ve deviation from Raoult's law
(2) n-heptane shows +ve deviation while ethanol shows –ve deviation from Raoult's law
(3) The solution formed is an ideal solution.
(4) The solutionis non-ideal, showing +ve deviation from Raoult's law
[AIEEE-2009]
Ans. (4)
(A) n–heptone : Non Polar
(B) Ethanol : Polar
$\mathrm{F}_{\mathrm{A}-\mathrm{B}}<\mathrm{F}_{\mathrm{A}-\mathrm{A}}, \mathrm{F}_{\mathrm{B}-\mathrm{B}} \Rightarrow+$ deviation
Q. Two liquids X and Y form an ideal solution. At 300K, vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mm Hg. At the same temperature, if 1 mol of Y is further added to this solution, vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mmHg) of X and Y in their pure states will be, respectively :-
(1) 400 and 600 (2) 500 and 600 (3) 200 and 300 (4) 300 and 400
[AIEEE-2009]
Ans. (1)
$550=\mathrm{P}_{\mathrm{A}}^{\circ} \times \frac{1}{4}+\mathrm{P}_{\mathrm{B}}^{\circ} \times \frac{3}{4}$
$560=\mathrm{P}_{\mathrm{A}}^{\circ} \times \frac{1}{5}+\mathrm{P}_{\mathrm{B}}^{\circ} \times \frac{4}{5}$
$\mathrm{P}_{\mathrm{A}}^{\circ}=400
\quad \mathrm{P}_{\mathrm{B}}^{\circ}=600$ torr
Q. On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of
the two liquid components (heptane and octane) are 105 kPa and 45 kPa respectively.
Vapour pressure of the solution obtained by mixing 25.0 of heptane and 35 g of octane
will be (molar mass of heptane = 100 g $\mathrm{mol}^{-1}$ and of octane = 114 g $\mathrm{mol}^{-1}$) :-
(1) 144.5 kPa (2) 72.0 kPa (3) 36.1 kPa (4) 96.2 kPa
[AIEEE-2010]
Ans. (2)
$\mathrm{P}_{\mathrm{A}}=\mathrm{P}_{\mathrm{A}}^{\circ} \mathrm{X}_{\mathrm{A}}=105 \times \frac{1 / 4}{1 / 4+0.307}$
$=105 \times 0.449=47.13 \mathrm{K} \mathrm{Pa}$
$\mathrm{P}_{\mathrm{B}}=\mathrm{P}_{\mathrm{B}}^{\circ} \mathrm{X}_{\mathrm{B}}=45 \times 0.551=24.795$
$\mathrm{P}_{\mathrm{T}}=\mathrm{P}_{\mathrm{A}}+\mathrm{P}_{\mathrm{B}}=71.925 \mathrm{atm}$
Q. If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the change in freezing point of water $\left(\Delta \mathrm{T}_{\mathrm{f}}\right)$, when 0.01 mol of sodium sulphate isdissolved in 1 kg of water, is $\left(\mathrm{K}_{\mathrm{f}}=1.86 \mathrm{K} \mathrm{kg} \mathrm{mol}^{-1}\right):$ :-
(1) 0.0186 K (2) 0.0372 K (3) 0.0558 K (4) 0.0744 K
[AIEEE-2010]
Ans. (3)
$\Delta \mathrm{T}_{\mathrm{f}}=\mathrm{i} \mathrm{k}_{\mathrm{f}} \cdot \mathrm{m}$
$=3 \times 1.86 \times 0.01 / 1$
$=0.0558 \mathrm{K}$
Q. The molality of a urea solution in which 0.0100g of urea, $\left.\left[\mathrm{NH}_{2}\right)_{2} \mathrm{CO}\right]$ is added to 0.3000 $\mathrm{dm}^{3}$ of water at STP is :-
(1) 0.555 m
(2) $5.55 \times 10^{-4} \mathrm{m}$
(3) 33.3 m
(4) $3.33 \times 10^{-2} \mathrm{m}$
[AIEEE-2011]
Ans. (2)
$\mathrm{m}=\frac{\mathrm{n}}{\mathrm{W}(\mathrm{kg})}=\frac{0.01 / 60}{0.3 \mathrm{kg}}=5.55 \times 10^{-4} \mathrm{mol} / \mathrm{kg}$
Q. A 5% solution of cane sugar (molar mass 342) is isotonic with 1% of a solution of an unknown solute. The molar mass of unknown solute in g/mol is :-
(1) 136.2 (2) 171.2 (3) 68.4 (4) 34.2
[AIEEE-2011]
Ans. (3)
$\pi_{\mathrm{c.s}}=\pi_{\mathrm{Unk}}$
$\left(\frac{\mathrm{n}}{\mathrm{V}}\right)_{\mathrm{c.s.}} \mathrm{RT}=\left(\frac{\mathrm{n}}{\mathrm{V}}\right)_{\mathrm{unk} .} \mathrm{RT}$
$\frac{5 \times 10}{342}=\frac{1 \times 10}{\mathrm{M}}$
M = 68.4 gm/mol
Q. Ethylene glycol is used as an antifreeze in a cold climate. Mass of ethylene glycol which should be added to 4 kg of water to prevent it from freezing at – $6^{\circ} \mathrm{C}$ will be :
$\left(\mathrm{K}_{\mathrm{f}} \text { for water }=1.86 \mathrm{K} \mathrm{kgmol}^{-1}, \text { and molar mass of ethylene glycol }=62 \mathrm{gmol}^{-1}\right)$
(1) 400.00 g (2) 304.60 g (3) 804.32 g (4) 204.30 g
[AIEEE-2011]
Ans. (3)
$6=1.86 \times \frac{\mathrm{w} / 62}{4} \Rightarrow \mathrm{w}=800 \mathrm{gm}$
Q. The degree of dissociation () of a weak electrolyte, AxBy is related to van't Hoff factor (i) by the expression :-
$(1) \alpha=\frac{\mathrm{x}+\mathrm{y}-1}{\mathrm{i}-1}$
(2) $\alpha=\frac{\mathrm{x}+\mathrm{y}+1}{\mathrm{i}-1}$
(3) $\alpha=\frac{\mathrm{i}-1}{(\mathrm{x}+\mathrm{y}-1)}$
(4) $\alpha=\frac{\mathrm{i}-1}{\mathrm{x}+\mathrm{y}+1}$
[AIEEE-2011]
Ans. (3)
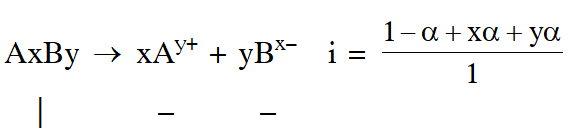
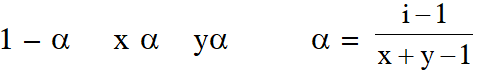
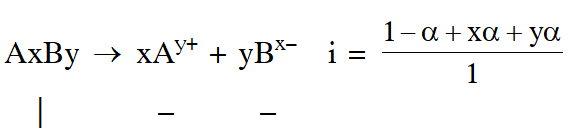
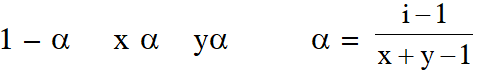
Q. $\mathrm{K}_{\mathrm{f}}$ for water is 1.86 K kg $\mathrm{mol}^{-1}$. If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol $\left(\mathrm{C}_{2} \mathrm{H}_{6} \mathrm{O}_{2}\right)$ must you add to get the freezing point of the solution lowered to –$-2.8^{\circ} \mathrm{C} ?$
(1) 27 g (2) 72 g (3) 93 g (4) 39 g
[AIEEE-2012]
Ans. (3)
$2.8=1.86 \times \frac{\mathrm{w} / 62}{1} \Rightarrow \mathrm{w}=93.33 \mathrm{gm}$
Q. A solution containing 0.85 g of $\mathrm{ZnCl}_{2}$ in 125.0 g of water freezes at $-0.23^{\circ} \mathrm{C}$ . The apparent degree of dissociation of the salt is :
($\mathbf{k}_{f}$ for water = 1.86 K kg $\mathrm{mol}^{-1}$, atomic mass ; Zn = 65.3 and Cl = 35.5)
(1) 1.36% (2) 2.47% (3) 73.5% (4) 7.35%
[Jee (Main)-2012 online]
Ans. (3)
$0.23=(1+2 \alpha) \times 1.86 \times \frac{0.85 / 134.5}{0.125}$
$\alpha=0.735=73.5 \%$
Q. Liquids A and B form an ideal solution. At $30^{\circ}$C, the total vapour pressure of a solution containing 1 mol of A and 2 moles of B is 250 mm Hg. The total vapour pressure becomes 300 mm Hg when 1 more mol of A is added to the first solution. The vapour pressures of pure A and B at the same temperature are
(1) 450, 150 mm Hg
(2) 250, 300 mm Hg
(3) 125, 150 mm Hg
(4) 150, 450 mm Hg
[Jee (Main)-2012 online]
Ans. (1)
$250=\mathrm{P}_{\mathrm{A}}^{0} \times \frac{1}{3}+\mathrm{P}_{\mathrm{B}}^{0} \times \frac{2}{3}$
$300=\mathrm{P}_{\mathrm{A}}^{0} \times \frac{1}{2}+\mathrm{P}_{\mathrm{B}}^{0} \times \frac{1}{2}$
$\mathrm{P}_{\mathrm{A}}^{0}=450 \mathrm{mm}$
$\mathrm{P}_{\mathrm{B}}^{0}=150 \mathrm{mm}$
Q. The freezing point of a 1.00 m aqueous solution of HF is found to be $-1.91^{\circ} \mathrm{C}$. The
freezing point constant of water, $\mathrm{K}_{\mathrm{f}}$, is 1.86 K kg $\mathrm{mol}^{-1}$. The percentage dissociation of HF at this concentration is
(1) 2.7% (2) 30% (3) 10% (4) 5.2%
[Jee (Main)-2012 online]
Ans. (1)
$\Delta \mathrm{T}_{\mathrm{f}}=\mathrm{i} \times \mathrm{K}_{\mathrm{f}} \times \mathrm{m}$
$1.91=(1+\alpha) \times 1.86 \times 1$
$\alpha=0.027$
Q. How many grams of methyl alcohol should be added to 10 litre tank of water to prevent its freezing at 268 K ?
$\left(\mathrm{K}_{f} \text { for water is } 1.86 \mathrm{K} \mathrm{kg} \mathrm{mol}^{-1}\right)$
(1) 899.04 g (2) 886.02 g (3) 868.06 g (4) 880.07 g
[Jee (Main)-2013 online]
Ans. (2)
$\Delta \mathrm{T}_{\mathrm{f}}=\mathrm{T}_{\mathrm{f}}^{0}-\mathrm{T}_{\mathrm{f}}=\mathrm{K}_{\mathrm{f}} \times \mathrm{m}$
$273.15-268=1.86 \times \frac{\mathrm{w} / 32}{10}$
$\mathrm{w}=886.02 \mathrm{g}$
Q. Vapour pressure of pure benzene is 119 torr and that of toluene is 37.0 torr at the same temperature. Mole fraction of toluene in vapour phase which is in equilibrium with a solution of benzene and toluene having a mole fraction of toluene 0.50, will be :
(1) 0.137 (2) 0.205 (3) 0.237 (4) 0.435
[Jee (Main)-2013 online]
Ans. (3)
Benzen $\rightarrow 4$
Toluene $\rightarrow B$
y $_{B}=\frac{P_{B}^{0} \times X_{B}}{P_{B}^{0} X_{B}+P_{A}^{0} X_{A}}=\frac{37 \times 0.5}{37 \times 0.5+119 \times 0.5}=0.237$
Q. A molecule M associates in a given solvent according to the equation M $(\mathrm{M})_{\mathrm{n}}$. For a certain concentration of M, the van’t Hoff factor was found to be 0.9 and the fraction of associated molecules was 0.2. The value of n is :
(1) 2 (2) 4 (3) 5 (4) 3
[Jee (Main)-2013 online]
Ans. (1)
$\mathrm{M}=\mathrm{M}_{\mathrm{n}}$
$1-0.2 \quad 0.2 / \mathrm{n}$
$0.9=\frac{1-0.2+0.2 / \mathrm{n}}{1}$
$0.9=0.8+\frac{0.2}{\mathrm{n}}$
$0.1=\frac{0.2}{\mathrm{n}}$
$\mathrm{n}=2$
Q. 12g of a nonvolatile solute dissolved in 108g of water produces the relative lowering of vapour pressure of 0.1. The molecular mass of the solute is :
(1) 60 (2) 80 (3) 40 (4) 20
[Jee (Main)-2013 online]
Ans. (4)
$\frac{\Delta \mathrm{P}}{\mathrm{P}^{0}}=0.1=\frac{12 / \mathrm{m}}{108 / 18} \Rightarrow \mathrm{m}=20$
Q. The molarity of a solution obtained by mixing 750 mL of 0.5(M)HCl with 250 mL of 2(M)HCl will be :-
(1) 0.875 M (2) 1.00 M (3) 1.75 M (4) 0.975 M
[Jee (Main)-2013]
Ans. (1)
$\mathrm{M}_{\mathrm{f}}=\frac{\mathrm{M}_{1} \mathrm{V}_{1}+\mathrm{M}_{2} \mathrm{V}_{2}}{\mathrm{V}_{1}+\mathrm{V}_{2}}=0.875 \mathrm{M}$
Q. The observed osmotic pressure for a 0.10 M solution of Fe$\left(\mathrm{NH}_{4}\right)_{2}\left(\mathrm{SO}_{4}\right)_{2}$ at $25^{\circ} \mathrm{C}$ is 10.8 atm. The expected and experimental (observed) values of Van't Hoff factor (i) will be respectively : $\left(\mathrm{R}=0.082 \mathrm{L} \mathrm{atm} \mathrm{k}^{-} \mathrm{mol}^{-1}\right)$
(1) 3 and 5.42 (2) 5 and 3.42 (3) 4 and 4.00 (4) 5 and 4.42
[Jee (Main)-2014 online]
Ans. (4)
$\pi_{\mathrm{ob}}=\mathrm{i} \frac{\mathrm{n}}{\mathrm{V}} \mathrm{RT}$
$10.8=\mathrm{i} \times 0.1 \times 0.082 \times 298$
$\mathrm{i}=4.42$
Q. For an ideal Solution of two components A and B, which of the following is true ?
(1) $\Delta \mathrm{H}_{\text {mixing }}<0$ (zero)
(2) $\mathrm{A}-\mathrm{A}, \mathrm{B}-\mathrm{B}$ and $\mathrm{A}-\mathrm{B}$ interactions are identical
(3) $\mathrm{A}-\mathrm{B}$ interaction is stronger than $\mathrm{A}-\mathrm{A}$ and $\mathrm{B}-\mathrm{B}$ interactions
(4) $\Delta \mathrm{H}_{\text {mixing }}>0$ (zero)
[Jee(Main)-2014 online]
Ans. (2)
$\Delta \mathrm{H}_{\operatorname{mix}}=0$
Q. Consider separate solution of $0.500 \mathrm{M} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{aq}), 0.100 \mathrm{MM} \mathrm{g}_{3}\left(\mathrm{PO}_{4}\right)_{2}(\mathrm{aq}), 0.250 \mathrm{M} \mathrm{KBr}(\mathrm{aq})$ and 0.125 M $\mathrm{Na}_{3} \mathrm{PO}_{4}(\mathrm{aq})$ at $25^{\circ} \mathrm{C}$. Which statement is true about these solutions, assuming all salts to be strong electrolytes ?
(1) 0.125 M $\mathrm{Na}_{3} \mathrm{PO}_{4}$ (aq) has the highest osmotic pressure.
(2) 0.500 M $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$ (aq) has the highest osmotic pressure.
(3) They all have the same osmotic pressure.
(4) 0.100 M $\mathrm{Mg}_{3}\left(\mathrm{PO}_{4}\right)_{2}$ (aq) has the highest osmotic pressure.
[Jee (Main)-2014]
Ans. (3)
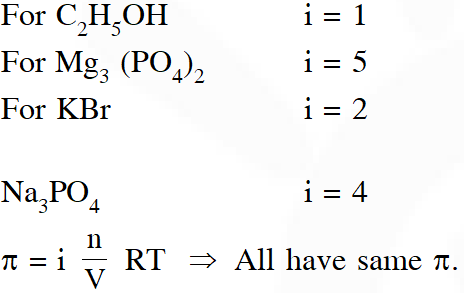
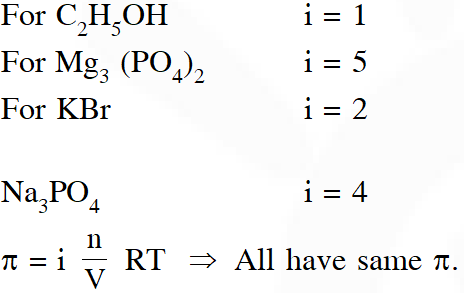
Q. Determination of the molar mass of acetic acid in benzene using freezing point depression is affected by :
(1) association
(2) dissociation
(3) complex formation
(4) partial ionization
[Jee (Main)-2015 online]
Ans. (1)
Acetic acid in non polar solvent (benzene) associates.
Q. A solution at $20^{\circ} \mathrm{C}$ is composed of 1.5 mol of benzene and 3.5 mol of toluene. If the vapour pressure of pure benzene and pure toluene at this temperature are 74.7 torr and 22.3 torr, respectively, then the total vapour pressure of the solution and the benzene mole fraction in equilibrium with it will be, respectively :
(1) 38.0 torr and 0.589
(2) 30.5 torr and 0.389
(3) 35.8 torr and 0.280
(4) 35.0 torr and 0.480
[Jee (Main)-2015 online]
Ans. (1)
$\begin{aligned} \mathrm{P}_{\mathrm{T}} &=\mathrm{P}_{\mathrm{A}}^{0} \mathrm{X}_{\mathrm{A}}+\mathrm{P}_{\mathrm{B}}^{0} \mathrm{X}_{\mathrm{B}} \\ &=747 \times \frac{1.5}{5}+22.3 \times \frac{3.5}{5} \\ &=38 \mathrm{torr} \end{aligned}$
Q. The vapour pressure of acetone at $20^{\circ}$C is 185 torr. When 1.2 g of non-volatile substance was dissolved in 100 g of acetone at $20^{\circ}$$20^{\circ}$C, its vapour pressure was 183 torr. The molar mass $\left(\mathrm{g} \mathrm{mol}^{-1}\right)$ of the substance is :
(1) 128 (2) 488 (3) 32 (4) 64
[Jee (Main)-2015]
Ans. (4)
$\begin{array}{rl}{\frac{185-183}{185}} & {=\frac{1.2 / \mathrm{m}}{100 / 58}} \\ {\mathrm{m}=64} & {\mathrm{gm} / \mathrm{mol}}\end{array}$
Q. For 1 molal aqueous solution of the following compounds, which one will show the highest freezing point ?
(1) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$
(2) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Cl} .2 \mathrm{H}_{2} \mathrm{O}$
(3) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{Cl}_{3}\right] \cdot 3 \mathrm{H}_{2} \mathrm{O}$
(4) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}$
[Jee (Main)-2018]
Ans. (3)
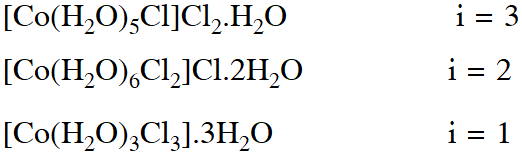
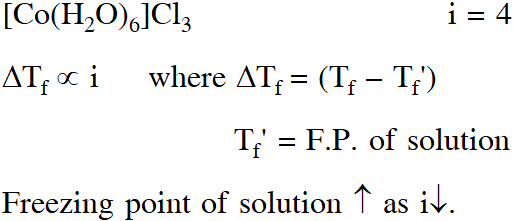
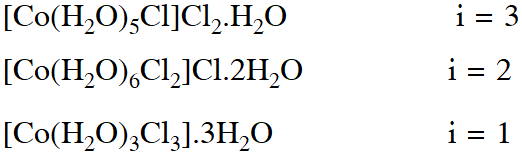
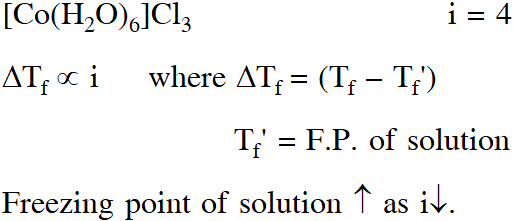
Comments
Harshu jatt
Dec. 25, 2023, 3:48 p.m.
Very good
But agr video solution sath me or hote to mja aata kyuki kuch question ese bhi the jinme video solution complusary tha
But almost is right ❤️
JEE Aspirent..........
July 11, 2023, 6:35 a.m.
Latest question can be more helpful .....
Please add them...
