JEE Main Previous Year Papers Questions of Chemistry with Solutions are available at eSaral. Practicing JEE Main chapter wise questions of Chemistry will help the JEE aspirants in realising the question pattern as well as help in analysing weak & strong areas.
Simulator
Previous Years AIEEE/JEE Main Question
Q. Which method of purification is represented by the following equation :
$\mathrm{Ti}(\mathrm{s})+2 \mathrm{I}_{2}(\mathrm{g}) \stackrel{\mathrm{s} 23 \mathrm{K}}{\longrightarrow} \mathrm{TiI}_{4}(\mathrm{g}) \stackrel{1700 \mathrm{K}}{\longrightarrow} \mathrm{Ti}(\mathrm{s})+2 \mathrm{I}_{2}(\mathrm{g})$
(1) Van Arkel
(2) Zone refining
(3) Cupellation
(4) Poling
[AIEEE-2012]
Ans. (1)
Explanation
Ti,Zr,Hg etc can be purified by Van Arkel's process
Q. The substance used as froth stabilisers in froth-floatation process is :
(1) Copper sulphate
(2) Aniline
(3) Sodium cyanide
(4) Potassium ethyl xanthate
[J-Mains-2012 (Online)]
Ans. (2)
Explanation
Aniline is used to stablises the forth.
Q. Which of the oxide groups among the following cannot be reduced by carbon :-
(1) $\mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{ZnO}$
(2) $\mathrm{PbO}, \mathrm{Fe}_{2} \mathrm{O}_{4}$
(3) $\mathrm{Cu}_{2} \mathrm{O}, \mathrm{SnO}_{2}$
(4) $\mathrm{CaO}, \mathrm{K}_{2} \mathrm{O}$
[J-Mains-2012 (Online)]
Ans. (4)
Explanation
More reactive metal such as K and "Ca" etc. can be used by electrolytic reduction method but
not by carbon reduction method.
Q. In Goldschmidt alumino thermic process which of the following reducing agents is used :
(1) Calcium
(2) Coke
(3) Sodium
(4) Al-powder
Ans. (4)
In alumino thermic process 'Al' metal is used as reducing agent.
Q. Calcination is the process in which :
(1) Ore is heated strongly below its melting point in the presence of excess of ait and is used
for the conversion of carbonates and hydrated oxide ores to their respective oxides.
(2) Ore is heated strongly below its melting point in the absence or limited supply of air and
is used for conversion of sulphide ores to their respective oxides
(3) Ore is heated strongly below its melting point either in the limited or absence of air
and is used to convert carbonates and hydrated oxide ores to their respective oxides
(4) Ore is heated strongly above its melting point in the limited supply of air to convert
sulphide ores to their respective oxides.
[JEE-MAINS 2014]
Ans. (3)
Calcination is carried out in absence of air.
Q. The metal that cannot be obtained by electrolysis of an aqueous solution of its salts is :
(1) Cu
(2) Cr
(3) Ag
(4) Ca
Ans. (4)
Explanation
"Ca" is more reactive metal, Which can be obtained by electrolysis of molten electrolyte of
$\mathrm{CaCl}_{2}$.But not in aqueous solution.
Q. The form of iron obtained from blast furnace is :
(1) Steel
(2) Wrought Iron
(3) Cast Iron
(4) Pig iron
[J-Mains-2014 (Online)]
Ans. (4)
Pig iron is obtained from blast furnace . Which is impurest form of iron.
Q. In the context of the Hall-Heroult process for the extreaction of Al, which of the following statements is false ?
(1) $\mathrm{Al}^{3+}$ is reduced at the cathode to form Al
(2) $\mathrm{Na}_{3}$ AlF_ $_{6}$ serves as the electrolyte
(3) $\mathrm{CO}$ and $\mathrm{CO}_{2}$ are produced in this process
(4) $\mathrm{Al}_{2} \mathrm{O}_{3}$ is mixed with $\mathrm{CaF}_{2}$ which lowers the melting point of the mixture and brings conductivity
[JEE-MAINS-2015]
Ans. (2)
Explanation
n the Hall-Heroult process 'Al' is reduced at cathode by electrolysis of molten electrolyte
$\mathrm{Al}_{2} \mathrm{O}_{3}$ but not $\mathrm{Na}_{3} \mathrm{AlF}_{6}$
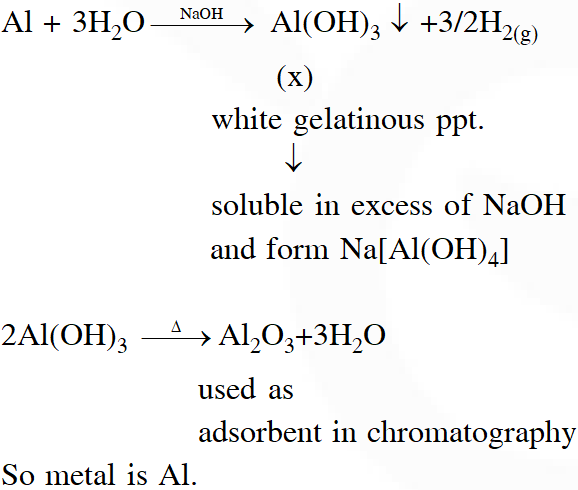
Q. When metal 'M' is treated with NaOH, a white gelatinous precipitate 'X' is obtained, which is soluble in excess of NaOH. Compound 'X' when heated strongly gives an oxide which is used in chromatography as an adsorbent. The metal 'M' is
(1) Ca
(2) Al
(3) Fe
(4) Zn
[JEE-MAINS-2018]
Ans. (2)
Explanation

Comments
Satiksha
Sept. 25, 2020, 12:38 p.m.
Thank you so much for this ...but can you put up more questions.
Satyam Trivedi
Sept. 11, 2020, 2:23 p.m.
Thank you sir
As I request to you sir please provide us more questions
NIHARIKA
Sept. 10, 2020, 4:04 p.m.
good , but some more questions which gains knowledge and useful for competitive tests
Poojapatel
Aug. 26, 2020, 12:37 p.m.
I need some extra questions sir
Like recent 2019 and 2020 questions
Likhitha
July 15, 2020, 10:48 p.m.
Good questions sir but we need some more they should be some what tricky
Sweety
June 17, 2020, 8:19 p.m.
Not only upto 2018 need 2019 also sir if possible keep before year paper bits sir.Till 2018 bits helped me a lot kindly keep 2019 too
