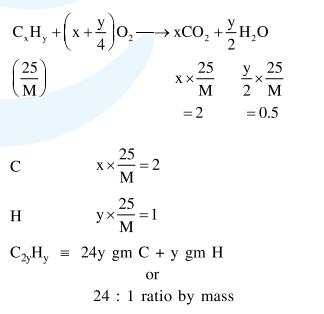
Question: $25 \mathrm{~g}$ of an unknown hydrocarbon upon burning produces $88 \mathrm{~g}$ of $\mathrm{CO}_{2}$ and $9 \mathrm{~g}$ of $\mathrm{H}_{2} \mathrm{O}$. This unknown hydrocarbon contains.
$20 \mathrm{~g}$ of carbon and $5 \mathrm{~g}$ of hydrogen
$24 \mathrm{~g}$ of carbon and $1 \mathrm{~g}$ of hydrogen
$18 \mathrm{~g}$ of carbon and $7 \mathrm{~g}$ of hydrogen
$22 \mathrm{~g}$ of carbon and $3 \mathrm{~g}$ of hydrogen
Correct Option: , 2
Solution: