Question:
A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The shape of this molecule is:
Correct Option: , 3
Solution:
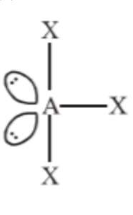
$\mathrm{sp}^{3} \mathrm{~d}$ hybridised
T-shaped
