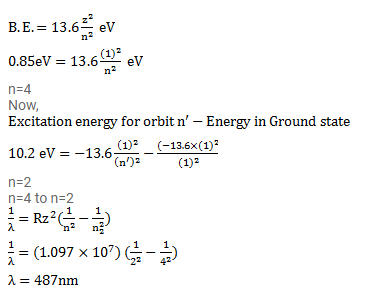
Question:
A hydrogen atom in a state having a binding energy of $0.85 \mathrm{eV}$ makes transition to a state with excitation energy $10.2 \mathrm{eV}$. Identify the quantum numbers $n$ of the upper and the lower energy states involved in the transition. Find the wavelength of the emitted radiation.
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
