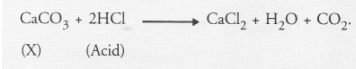
A metal carbonate (X) on reacting with an acid gives a gas which when passed through a solution (Y) gives the carbonate back. On the other hand, a
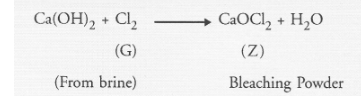
gas (G) that is obtained at anode during electrolysis of brine is passed on dry substance (Y). It gives a compound (Z), used for disinfecting drinking
water. Identity X, Y, G and Z.
The gas (G) obtained at anode during the electrolysis of brine is chlorine. The compound (Z) used for disinfecting drinking water is bleaching
powder. It is formed on reacting chlorine with dry slaked lime i.e., Ca(OH)2. It is denoted as ‘Y’ This means that the metal carbonate ‘X’ is calcium
carbonate. Upon heating, it evolves CO2 gas which gives back ‘X’ on reacting with calcium hydroxide. The chemical reactions involved are listed :

Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
