A non-metal A which is the largest constituent of air, when heated with H2 in 1 : 3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with
O2 it gives an oxide C. If this oxide is passed into water in the presence of air, it gives an acid D which acts as a strong oxidising agent.
(a) Identify A, B, C and D.
(b) To which group of periodic table does this non-metal belong ?
(a) The available information suggests that the non-metal A is nitrogen (N) and its molecular form is N2. It is the major constituent of air (about 79 per
cent by volume). It reacts with H2 upon heating m the presence of iron catalyst (Fe) to form ammonia (NH3) gas(g)
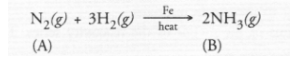
Upon heating with oxygen, nitrogen forms intially nitric oxide (NO) and then nitrogen dioxide (C). The latter reacts with water in the presence of
oxygen to form nitric acid (D). The acid acts as strong oxidising agent.
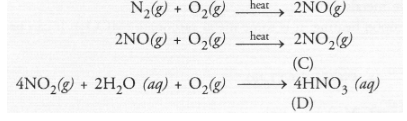
(b) The element nitrogen is the first member of group 15 of Modern periodic table.
