Question:
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin.
The plots at different values of pressure are shown in the figure. Which , of the following order of pressure is correct for this gas?
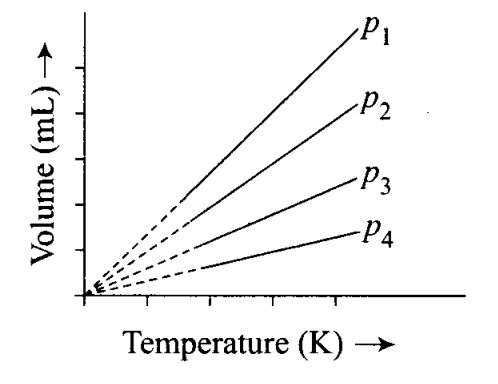
(a)P1 >P2 >P3 >P4
(b) P1 =p2 =p3 =p4
(c) P1
(d) px
Solution:
(c) At a particular temperature pV= constant.
Thus,
P1V1 =P2V2=P3V3=P4V4
As V1> V2 > V3 > V 4, therefore, P1
