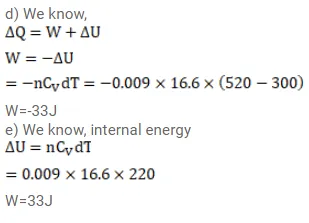Question:
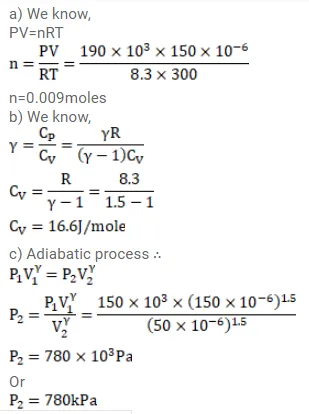
A sample of an ideal gas $(\gamma=1.5)$ is compressed adiabatically from a volume of $150 \mathrm{~cm}^{3}$ to $50 \mathrm{~cm}^{3}$. The initial pressure and the initial temperature are $150 \mathrm{kPa}$ and $300 \mathrm{~K}$. Find (a) the number of moles of the gas in the sample, (b) the molar heat capacity at constant volume, (c) the final pressure and temperature, (d) the work done by the gas in the process and (e) the change in internal energy of the gas.
Solution: