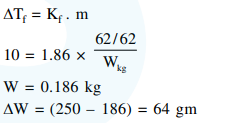Question:
A solution containing $62 \mathrm{~g}$ ethylene glycol in $250 \mathrm{~g}$ water is cooled to $-10^{\circ} \mathrm{C}$. If $\mathrm{K}_{\mathrm{f}}$ for water is $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$, the amount of water (in $\mathrm{g}$ ) separated as ice is :
Correct Option: , 4
Solution: