Question:
A vessel of 120 mL capacity contains a certain amount of gas at 35 °C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35 °C. What would be its pressure?
Solution:
Given,
Initial pressure, p1 = 1.2 bar
Initial volume, V1 = 120 mL
Final volume, V2 = 180 mL
Since the temperature remains constant, the final pressure (p2) can be calculated using Boyle’s law.
According to Boyle’s law,
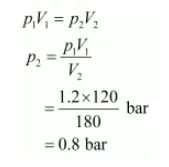
Therefore, the pressure would be 0.8 bar.
