Question:
A vessel of 120 mL capacity contains a certain amount of gas at 35 °C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35 °C. What would be its pressure?
Solution:
Given,
Initial pressure, p1 = 1.2 bar
Initial volume, V1 = 120 mL
Final volume, V2 = 180 mL
Since the temperature remains constant, the final pressure (p2) can be calculated using Boyle’s law.
According to Boyle’s law,
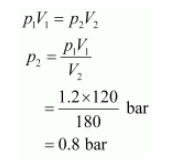
Therefore, the pressure would be 0.8 bar.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
