Question:
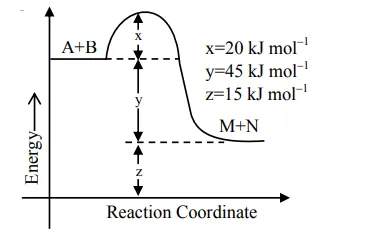
According to the following figure, the magnitude of the enthalpy change of the reaction
$\mathrm{A}+\mathrm{B} \rightarrow \mathrm{M}+\mathrm{N}$ in $\mathrm{kJ} \mathrm{mol}^{-1}$
is equal to____________(Integer answer)
Solution:
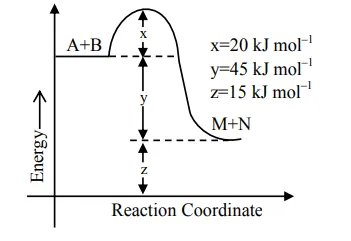
$\Delta \mathrm{H}=\mathrm{E}_{\mathrm{a}_{\mathrm{f}}}-\mathrm{E}_{\mathrm{a}_{\mathrm{b}}}$
$=20-65$
$=-45 \mathrm{KJ} / \mathrm{mol}$
$|\Delta \mathrm{H}|=45 \mathrm{KJ} / \mathrm{mol}$
