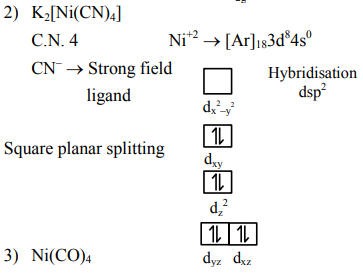
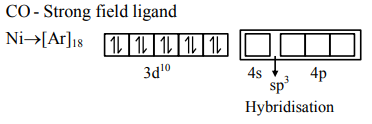
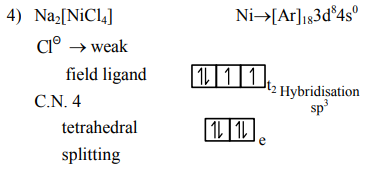
Question:
According to the valence bond theory the hybridization of central metal atom is $\mathrm{dsp}^{2}$ for which one of the following compounds?
Correct Option: , 2
Solution:
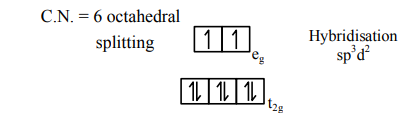
1) $\mathrm{NiCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$
$\mathrm{Ni}^{+2} \rightarrow[\mathrm{Ar}]_{18} 3 \mathrm{~d}^{8} 4 \mathrm{~s}^{0}$
C.N. $=6$ octahedral