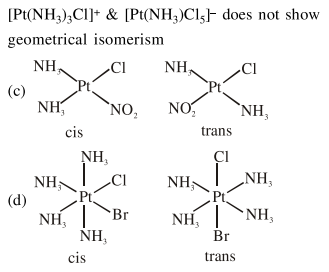
Question:
Among (a) - (d) the complexes that can display geometrical isomerism are :
(a) $\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{3} \mathrm{Cl}\right]^{+}$
(b) $\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right) \mathrm{Cl}_{5}\right]^{-}$
(c) $\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}\left(\mathrm{NO}_{2}\right)\right]$
(d) $\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{ClBr}\right]^{2+}$
Correct Option: , 4
Solution: