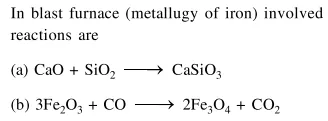Question:
Among the reactions (a) - (d), the reaction(s) that does/do not occur in the blast furnace during the extraction of iron is/are :
(a) $\mathrm{CaO}+\mathrm{SiO}_{2} \rightarrow \mathrm{CaSiO}_{3}$
(b) $3 \mathrm{Fe}_{2} \mathrm{O}_{3}+\mathrm{CO} \rightarrow 2 \mathrm{Fe}_{3} \mathrm{O}_{4}+\mathrm{CO}_{2}$
(c) $\mathrm{FeO}+\mathrm{SiO}_{2} \rightarrow \mathrm{FeSiO}_{3}$
(d) $\mathrm{FeO} \rightarrow \mathrm{Fe}+\frac{1}{2} \mathrm{O}_{2}$
Correct Option: 1
Solution: