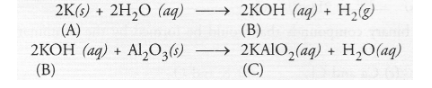Question:
An alkali metal A gives a compound B (molecular mass = 56) on reacting with water. The compound ‘B’ gives a soluble compound ‘C’ on treatment with
aluminium oxide. Identify A, B and C and give the reaction involved.
Solution:
As explained under Question 42, the alkali metal A is potassium (K) and on reacting with water, it forms a compound ‘B’ which is potassium
hydroxide. This upon reacting with aluminium oxide (Al2O3) forms potassium metaluminate ‘C’.