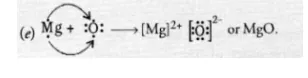Question:
An element placed in 2nd group and 3rd Period of the Periodic Table, burns in the presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in the presence of air
(d) Write a balanced equation when this oxide is dissolved in water
(e) Draw the electron dot structure for the formation of this oxide
Solution:
(a) The element is magnesium (Mg).
(b) The electronic configuration is 2, 8, 2.
(c) Magnesium burns in oxygen (air) to form magnesium oxide which is of basic nature.
2Mg(s) + O2(g) ————-> 2MgO(s)
(d) Magnesium hydroxide is formed.
MgO(s) + H2O(aq) ————> Mg(OH)2(s)