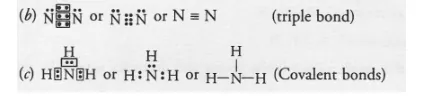Question:
An element X of group 15 exists as diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound,
ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does it have ?
(b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it ?
(c) Draw the electron dot structure for ammonia. What type of bonds is formed in it ?
Solution:
(a) The available information suggests that the element X is nitrogen (N) and exists in diatomic form as N,
Electronic configuration of N(Z = 7) ; 2, 5. It has five valence electrons.