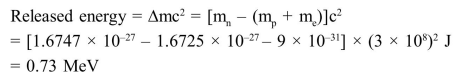Question:
Assume that a neutron breaks into a proton and an electron. The energy released during this process is:
(Mass of neutron $=1.6747 \times 10^{-27} \mathrm{~kg}$
Mass of proton $=1.6725 \times 10^{-27} \mathrm{~kg}$
Mass of electron $=9 \times 10^{-31} \mathrm{~kg}$ )
Correct Option: , 2
Solution: