Question:
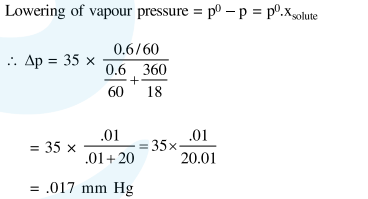
At room temperature, a dilute soluton of urea is prepared by dissolving $0.60 \mathrm{~g}$ of urea in $360 \mathrm{~g}$ of water. If the vapour pressure of pure water at this temperature is $35 \mathrm{mmHg}$, lowering of vapour pressure will be (molar mass of urea $=60 \mathrm{~g} \mathrm{~mol}^{-1}$ ):-
Correct Option: , 3
Solution: