Question.
Calculate the mass of sodium acetate $\left(\mathrm{CH}_{3} \mathrm{COONa}\right)$ required to make $500 \mathrm{~mL}$ of $0.375$
molar aqueous solution. Molar mass of sodium acetate is $82.0245 \mathrm{~g} \mathrm{~mol}^{-1}$
Calculate the mass of sodium acetate $\left(\mathrm{CH}_{3} \mathrm{COONa}\right)$ required to make $500 \mathrm{~mL}$ of $0.375$
molar aqueous solution. Molar mass of sodium acetate is $82.0245 \mathrm{~g} \mathrm{~mol}^{-1}$
Solution:
0.375 M aqueous solution of sodium acetate
≡ 1000 mL of solution containing 0.375 moles of sodium acetate
Number of moles of sodium acetate in 500 mL
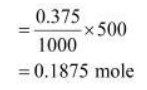
Molar mass of sodium acetate $=82.0245 \mathrm{~g}$ mole $^{-1}$ (Given)
$\therefore$ Required mass of sodium acetate $=\left(82.0245 \mathrm{~g} \mathrm{~mol}^{-1}\right)(0.1875 \mathrm{~mole})$
$=15.38 \mathrm{~g}$
0.375 M aqueous solution of sodium acetate
≡ 1000 mL of solution containing 0.375 moles of sodium acetate
Number of moles of sodium acetate in 500 mL
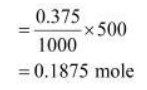
Molar mass of sodium acetate $=82.0245 \mathrm{~g}$ mole $^{-1}$ (Given)
$\therefore$ Required mass of sodium acetate $=\left(82.0245 \mathrm{~g} \mathrm{~mol}^{-1}\right)(0.1875 \mathrm{~mole})$
$=15.38 \mathrm{~g}$
