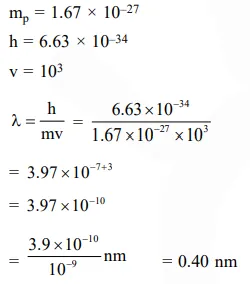Question:
Calculate the wavelength (in nanometer) associated with a proton moving at $1.0 \times 10^{3} \mathrm{~ms}^{-1}$ (Mass of proton $=1.67 \times 10^{-27} \mathrm{~kg}$ and $\mathrm{h}=6.63 \times 10^{-34} \mathrm{Js}$ ) :-
Correct Option: , 4
Solution: