Question:
Compound(s) which will liberate carbon dioxide with sodium bicarbonate solution is/are:
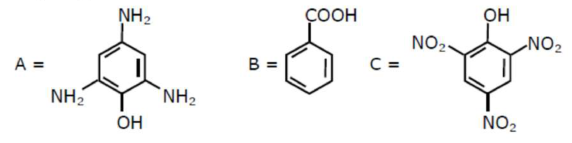
Correct Option: 1
Solution:
Compounds which are more acidic then $\mathrm{H}_{2} \mathrm{CO}_{3}$, gives $\mathrm{CO}_{2}$ gas on reaction with $\mathrm{NaHCO}_{3} .$ Compound $\mathrm{B}$ i.e. $\mathrm{Benzoic}$ acid and compound $\mathrm{C}$ i.e. picric acid both are more acidic than $\mathrm{H}_{2} \mathrm{CO}_{3}$.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
