Question:
Compounds ‘A’ and ‘B’ react according to the following chemical equation.
A (g) + 2 B (g) → 2C (g)
The concentration of either ‘A’ or ‘B’ was changed keeping the concentrations of
one of the reactants constant and rates were measured as a function of initial
concentration. Following results were obtained. Choose the correct option for
the rate equations for this reaction.
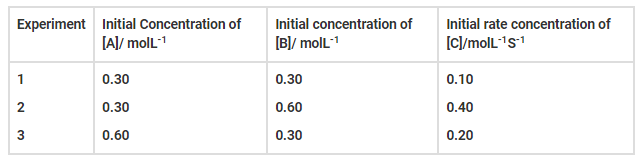
(i) Rate = k [A]2[B]
(ii) Rate = k [A] [B]2
(iii) Rate = k [A] [B]
(iv) Rate = k [A]2[B]0
Solution:
Option (ii) Rate = k [A] [B]2 is the answer.
