Question:
Consider the complex ions,
$\operatorname{trans}-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}(\mathrm{A})$ and
cis- $\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}(\mathrm{B})$. The correct statement regarding them is :
Correct Option: 4,
Solution:
(A) $\operatorname{trans}-\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}$
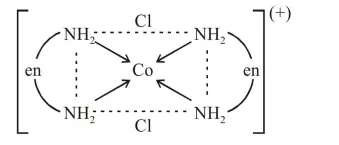
$\Rightarrow$ (A) is trans form and shows plane of symmetry which is optically inactive (not optically active)
(B) cis- $\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}$
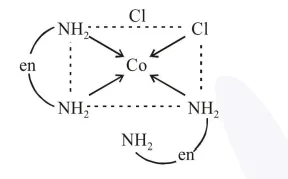
$\Rightarrow$ (B) is cis form and does not shows plane of symmetry, hence it is optically active.
