Question:
Consider the given plots for a reaction obeying Arrhenius equation $\left(\begin{array}{lll}0 & \left.{ }^{\circ} \mathrm{C}<\mathrm{T}<300{ }^{\circ} \mathrm{C}\right) \text { : }\end{array}\right.$ ( $\mathrm{K}$ and $\mathrm{E}_{\mathrm{a}}$ are rate constant and activation energy, respectively)
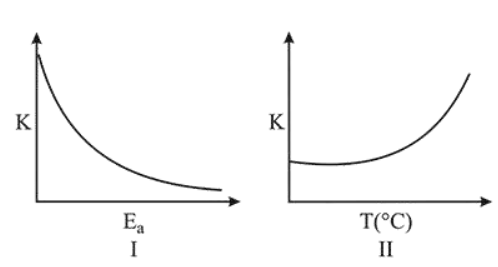
Choose the correct option:
Correct Option: , 2
Solution:
From Arrhenius equation,
$K=A e^{-E_{a} / R T}$
So, as $\mathrm{E}_{\mathrm{a}}$ increases, $e^{-E_{a} / R T}$ decreases, $K$ decreases
and as T increases, $\frac{E_{a}}{R T}$ decreases, $e^{-E_{a} / R T}$ increases,
