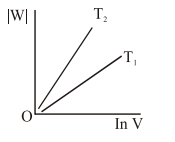
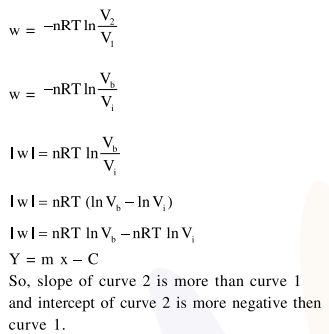Question:
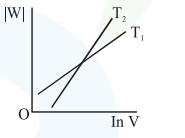
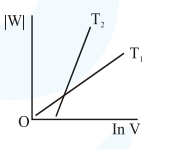
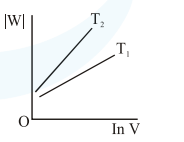
Consider the reversible isothermal expansion of an ideal gas in a closed system at two different temperatures $\mathrm{T}_{1}$ and $\mathrm{T}_{2}\left(\mathrm{~T}_{1}<\mathrm{T}_{2}\right)$. The correct graphical depiction of the dependence of work done (w) on the final volume (V) is:
Correct Option: , 2
Solution: