Question:
Define the bond length.
Solution:
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
Bond lengths are expressed in terms of Angstrom $\left(10^{-10} \mathrm{~m}\right)$ or picometer
$\left(10^{-12} \mathrm{~m}\right)$ and are measured by spectroscopic $X$-ray diffractions and electron-diffraction techniques.
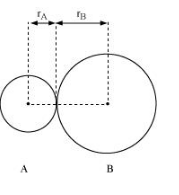
In an ionic compound, the bond length is the sum of the ionic radii of the constituting atoms $\left(d=r_{+}+r_{-}\right)$. In a covalent compound, it is the sum of their covalent radii ( $d=r_{A}+r_{B}$ ).
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
