Question.
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass
Solution:
% of iron by mass = 69.9 % [Given]
% of oxygen by mass = 30.1 % [Given] Relative
moles of iron in iron oxide
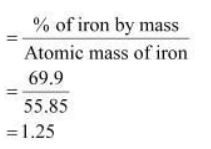
Relative moles of oxygen in iron oxide
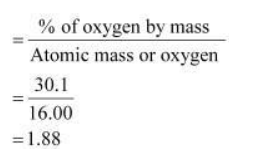
Simplest molar ratio of iron to oxygen
= 1.25: 1.88
= 1: 1.5
= 2: 3
$\therefore$ The empirical formula of the iron oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3}$.
% of iron by mass = 69.9 % [Given]
% of oxygen by mass = 30.1 % [Given] Relative
moles of iron in iron oxide
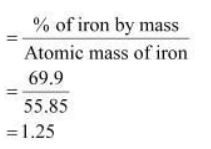
Relative moles of oxygen in iron oxide
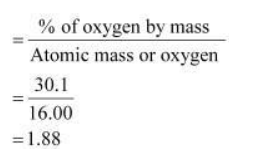
Simplest molar ratio of iron to oxygen
= 1.25: 1.88
= 1: 1.5
= 2: 3
$\therefore$ The empirical formula of the iron oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3}$.
