Question.
Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are $69.9$ and $30.1$ respectively. Given that the molar mass of the oxide is $159.69 \mathrm{~g} \mathrm{~mol}^{-1}$.
Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are $69.9$ and $30.1$ respectively. Given that the molar mass of the oxide is $159.69 \mathrm{~g} \mathrm{~mol}^{-1}$.
Solution:
Mass percent of iron (Fe) = 69.9% (Given)
Mass percent of oxygen (O) = 30.1% (Given)
Number of moles of iron present in the oxide $=\frac{69.90}{55.85}$
= 1.25
Number of moles of oxygen present in the oxide $=\frac{30.1}{16.0}$
= 1.88
Ratio of iron to oxygen in the oxide
$=1.25: 1.88$ $=\frac{1.25}{1.25}: \frac{1.88}{1.25}$
= 1 : 1.5
= 2 : 3
$\therefore$ The empirical formula of the oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3} .$
Empirical formula mass of $\mathrm{Fe}_{2} \mathrm{O}_{3}=[2(55.85)+3(16.00)]$ g Molar
mass of $\mathrm{Fe}_{2} \mathrm{O}_{3}=159.69 \mathrm{~g}$
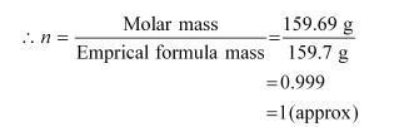
Molecular formula of a compound is obtained by multiplying the empirical formula with $n$.
Thus, the empirical formula of the given oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3}$ and $n$ is 1 .
Hence, the molecular formula of the oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3} .$
Mass percent of iron (Fe) = 69.9% (Given)
Mass percent of oxygen (O) = 30.1% (Given)
Number of moles of iron present in the oxide $=\frac{69.90}{55.85}$
= 1.25
Number of moles of oxygen present in the oxide $=\frac{30.1}{16.0}$
= 1.88
Ratio of iron to oxygen in the oxide
$=1.25: 1.88$ $=\frac{1.25}{1.25}: \frac{1.88}{1.25}$
= 1 : 1.5
= 2 : 3
$\therefore$ The empirical formula of the oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3} .$
Empirical formula mass of $\mathrm{Fe}_{2} \mathrm{O}_{3}=[2(55.85)+3(16.00)]$ g Molar
mass of $\mathrm{Fe}_{2} \mathrm{O}_{3}=159.69 \mathrm{~g}$
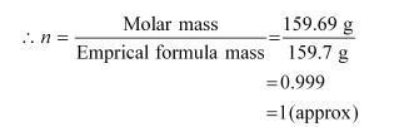
Molecular formula of a compound is obtained by multiplying the empirical formula with $n$.
Thus, the empirical formula of the given oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3}$ and $n$ is 1 .
Hence, the molecular formula of the oxide is $\mathrm{Fe}_{2} \mathrm{O}_{3} .$
