Equilibrium constant, Kc for the reaction
$\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \longleftrightarrow 2 \mathrm{NH}_{3}(\mathrm{~g})$ at $500 \mathrm{~K}$ is $0.061$
At a particular time, the analysis shows that composition of the reaction mixture is $3.0 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~N}_{2}, 2.0 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{H}_{2}$ and $0.5 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{NH}_{3}$. Is the reaction at equilibrium? If not in which direction does the reaction tend to proceed to reach equilibrium?
The given reaction is:
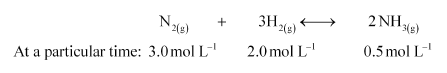
Now, we know that,
$Q_{\mathrm{C}}=\frac{\left[\mathrm{NH}_{3}\right]^{2}}{\left[\mathrm{~N}_{2}\right]\left[\mathrm{H}_{2}\right]^{3}}$
$=\frac{(0.5)^{2}}{(3.0)(2.0)^{3}}$
$=0.0104$
It is given that $K_{\mathrm{C}}=0.061$.
Since $Q_{C} \neq K_{C}$, the reaction is not at equilibrium.
Since $Q_{\mathrm{C}}
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
