Question:
Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar.
Solution:
The Lewis structure for BeH2 is as follows:
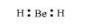
There is no lone pair at the central atom (Be) and there are two bond pairs. Hence, BeH2 is of the type AB2. It has a linear structure.
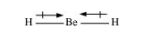
Dipole moments of each H–Be bond are equal and are in opposite directions. Therefore, they nullify each other. Hence, BeH2 molecule has zero dipole moment.
