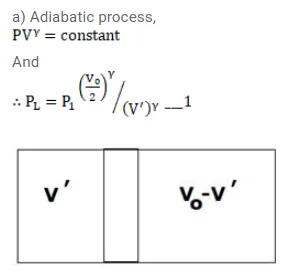
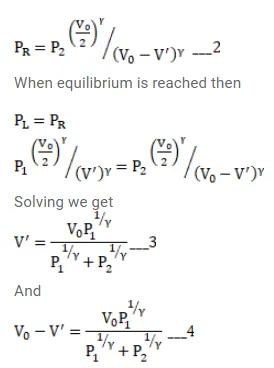Question:
Fig shows an adiabatic cylindrical tube of volume $V_{0}$ divided in two parts by a frictionless adiabatic separator. Initially, the separator is kept in the middle, an ideal gas at pressure $\mathrm{p}_{1}$ and temperature $\mathrm{T}_{1}$ is injected into the left part and another ideal gas at pressure $p_{2}$ and temperature $T_{2}$ is injected into the right part
$\left(C_{p} / C_{v}=\gamma\right)$ is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find
(a) the volumes of the two parts,
(b) the heat given to the gas in the left part and
(c) the final common pressure of the gases.
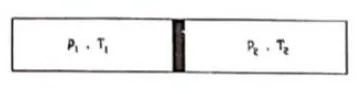
Solution: