Question.
Following results are observed when sodium metal is irradiated with different wavelengths. Calculate (a) threshold wavelength and, (b) Planck’s constant
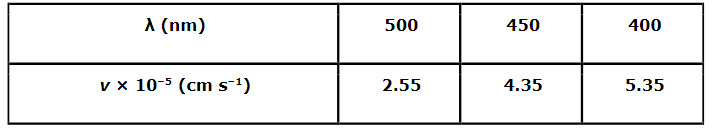
Following results are observed when sodium metal is irradiated with different wavelengths. Calculate (a) threshold wavelength and, (b) Planck’s constant

Solution:
(a) Assuming the threshold wavelength to be $\lambda_{0} \mathrm{~nm}\left(=\lambda_{0} \times 10^{-9} \mathrm{~m}\right)$, the kinetic energy of the radiation is given as:
$\mathrm{h}\left(v-v_{0}\right)=\frac{1}{2} m v^{2}$
Three different equalities can be formed by the given value as:
$h c\left(\frac{1}{\lambda}-\frac{1}{\lambda_{0}}\right)=\frac{1}{2} m v^{2}$
$h c\left(\frac{1}{500 \times 10^{9}}-\frac{1}{\lambda_{0} \times 10^{-9} \mathrm{~m}}\right)=\frac{1}{2} m\left(2.55 \times 10^{+5} \times 10^{-2} \mathrm{~ms}^{-1}\right)$
$\frac{h c}{10^{-9} m}\left[\frac{1}{500}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(2.55 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (1)
Similarly,
$\frac{h c}{10^{-9} \mathrm{~m}}\left[\frac{1}{450}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(3.45 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (2)
$\frac{h c}{10^{-0} \mathrm{~m}}\left[\frac{1}{400}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(5.35 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (3)
Dividing equation (3) by equation (1):
$\frac{\left[\begin{array}{c}\lambda_{0}-400 \\ 400 \lambda_{0}\end{array}\right]}{\left[\begin{array}{c}\lambda_{0}-500 \\ 500 \lambda_{0}\end{array}\right]}=\frac{\left(5.35 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}}{\left(2.55 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=\left(\frac{5.35}{2.55}\right)^{2}=\frac{28.6225}{6.5025}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=4.40177$
$17.6070 \lambda_{0}-5 \lambda_{0}=8803.537-2000$
$\lambda_{0}=\frac{6805.537}{12.607}$
$\lambda_{0}=539.8 \mathrm{~nm}$
$\lambda_{0}=540 \mathrm{~nm}$
So, threshold wavelength $\left(\lambda_{0}\right)=540 \mathrm{~nm}$
Note: part (b) of the question is not done due to the incorrect values of velocity given in the question.
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=\left(\frac{5.35}{2.55}\right)^{2}=\frac{28.6225}{6.5025}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=4.40177$
$17.6070 \lambda_{0}-5 \lambda_{0}=8803.537-2000$
$\lambda_{0}=\frac{6805.537}{12.607}$
$\lambda_{0}=539.8 \mathrm{~nm}$
$\lambda_{0}=540 \mathrm{~nm}$
(a) Assuming the threshold wavelength to be $\lambda_{0} \mathrm{~nm}\left(=\lambda_{0} \times 10^{-9} \mathrm{~m}\right)$, the kinetic energy of the radiation is given as:
$\mathrm{h}\left(v-v_{0}\right)=\frac{1}{2} m v^{2}$
Three different equalities can be formed by the given value as:
$h c\left(\frac{1}{\lambda}-\frac{1}{\lambda_{0}}\right)=\frac{1}{2} m v^{2}$
$h c\left(\frac{1}{500 \times 10^{9}}-\frac{1}{\lambda_{0} \times 10^{-9} \mathrm{~m}}\right)=\frac{1}{2} m\left(2.55 \times 10^{+5} \times 10^{-2} \mathrm{~ms}^{-1}\right)$
$\frac{h c}{10^{-9} m}\left[\frac{1}{500}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(2.55 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (1)
Similarly,
$\frac{h c}{10^{-9} \mathrm{~m}}\left[\frac{1}{450}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(3.45 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (2)
$\frac{h c}{10^{-0} \mathrm{~m}}\left[\frac{1}{400}-\frac{1}{\lambda_{0}}\right]=\frac{1}{2} m\left(5.35 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}$ (3)
Dividing equation (3) by equation (1):
$\frac{\left[\begin{array}{c}\lambda_{0}-400 \\ 400 \lambda_{0}\end{array}\right]}{\left[\begin{array}{c}\lambda_{0}-500 \\ 500 \lambda_{0}\end{array}\right]}=\frac{\left(5.35 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}}{\left(2.55 \times 10^{+3} \mathrm{~ms}^{-1}\right)^{2}}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=\left(\frac{5.35}{2.55}\right)^{2}=\frac{28.6225}{6.5025}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=4.40177$
$17.6070 \lambda_{0}-5 \lambda_{0}=8803.537-2000$
$\lambda_{0}=\frac{6805.537}{12.607}$
$\lambda_{0}=539.8 \mathrm{~nm}$
$\lambda_{0}=540 \mathrm{~nm}$
So, threshold wavelength $\left(\lambda_{0}\right)=540 \mathrm{~nm}$
Note: part (b) of the question is not done due to the incorrect values of velocity given in the question.
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=\left(\frac{5.35}{2.55}\right)^{2}=\frac{28.6225}{6.5025}$
$\frac{5 \lambda_{0}-2000}{4 \lambda_{0}-2000}=4.40177$
$17.6070 \lambda_{0}-5 \lambda_{0}=8803.537-2000$
$\lambda_{0}=\frac{6805.537}{12.607}$
$\lambda_{0}=539.8 \mathrm{~nm}$
$\lambda_{0}=540 \mathrm{~nm}$
