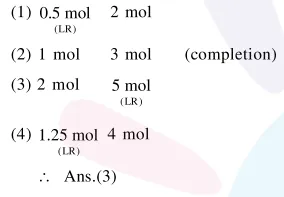
Question:
For a reaction,
$\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NH}_{3}(\mathrm{~g})$
identify dihydrogen $\left(\mathrm{H}_{2}\right)$ as a limiting reagent in the following reaction mixtures.
Correct Option: , 3
Solution:
$\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NH}_{3}(\mathrm{~g})$